Raccoon parvovirus enteritis and canine distemper dual inactivated vaccine and preparation method thereof
A dual inactivated vaccine and parvovirus technology, which is applied in the field of veterinary biological products, can solve the problems of poor immunogenicity of inactivated vaccines, increase the workload of farmers, and strong virulence of live vaccines, etc., to achieve safety and Good immunogenicity, no need for a large number of production personnel, and the effect of automated culture methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] 1) Preparation of VP2 protein of raccoon dog parvovirus: select vigorously growing Sf9 cells to inoculate a cell culture tank for full suspension culture, and the inoculation density is 1.5×10 6 ~2.0×10 6 cells / ml, cultured at 27°C, when the density of Sf9 cells in the cell culture tank reached 1.5×10 6 ~3.0×10 6Cells / ml, inoculate the recombinant baculovirus RDPV-SN1 strain at MOI=0.2~2.0, culture at 27°C for 72~96h, harvest the cell culture, add 2% BEI solution to it, make the final concentration of BEI 0.1%, After mixing, it was inactivated at 37 °C for 48 hours, then 50% sodium thiosulfate solution was added to the cell culture to make the final concentration 0.2%, and the inactivation was terminated after stirring for 1 hour; after sterility and inactivation tests, it was obtained Raccoon dog parvovirus VP2 protein, hemagglutination titer to 1% porcine red blood cells ≥ 1:2048, stored at 2-8°C for future use;
[0075] 2) Preparation of inactivated antigen of can...
Embodiment 1
[0086] Example 1——Research on suspension culture process of recombinant baculovirus RDPV-SN1 strain
[0087] 1. Research on the culture technology of the recombinant baculovirus RDPV-SN1 strain in the flask
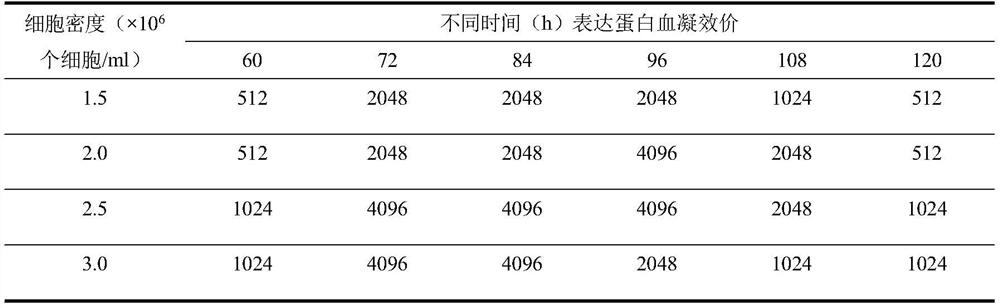
[0088] (1) Effect of cell density on hemagglutination titer of expressed protein
[0089] When the Sf9 cell density is 1.5 × 10 6 cells / ml, 2.0×10 6 cells / ml, 2.5×10 6 cells / ml, 3.0×10 6 When cells / ml, the recombinant baculovirus RDPV-SN1 strain was inoculated at MOI=0.5, and samples were taken at 60h, 72h, 84h, 96h, 108h, and 120h after inoculation to determine the hemagglutination titer of the expressed protein.
[0090] Results The recombinant baculovirus RDPV-SN1 strain was inoculated into Sf9 cells with different cell densities, and the Sf9 cell density was 1.5×10 when inoculated. 6 , 2.0×10 6 , 2.5×10 6 , 3.0×10 6 There was no significant difference in the hemagglutination titer of the expressed protein at 72h after inoculation, and the hemagglutination tite...
Embodiment 2
[0111] Embodiment 2——The research on suspension culture technology of canine distemper virus CDV-11 strain
[0112] 1. Study on the culture technology of canine distemper virus CDV-11 strain 7L cell culture tank
[0113] (1) Microcarrier preparation and sterilization 2+ , Mg 2+ PBS (0.1 mol / L, pH 7.2, the same below) was washed 2 to 3 times, and PBS was added to sterilize at 121 °C for 30 min. After sterilization, discard the PBS and add MEM cell culture medium containing 8% newborn bovine serum at 36.5°C, containing 5% CO. 2 Place in the incubator for 4h.
[0114] (2) Vero cell microcarrier suspension culture Select Vero spinner flask cells with good morphology and vigorous growth, digest with EDTA-trypsin dispersion to prepare cell suspension, inoculate a stirring cell culture flask, rotate speed 30r / min, and cell density is 4.5×10 5 cells / ml, the microcarrier concentration was 4 g / L, and the culture temperature was 36.5°C to 37.5°C.
[0115] (3) Influence of culture t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com