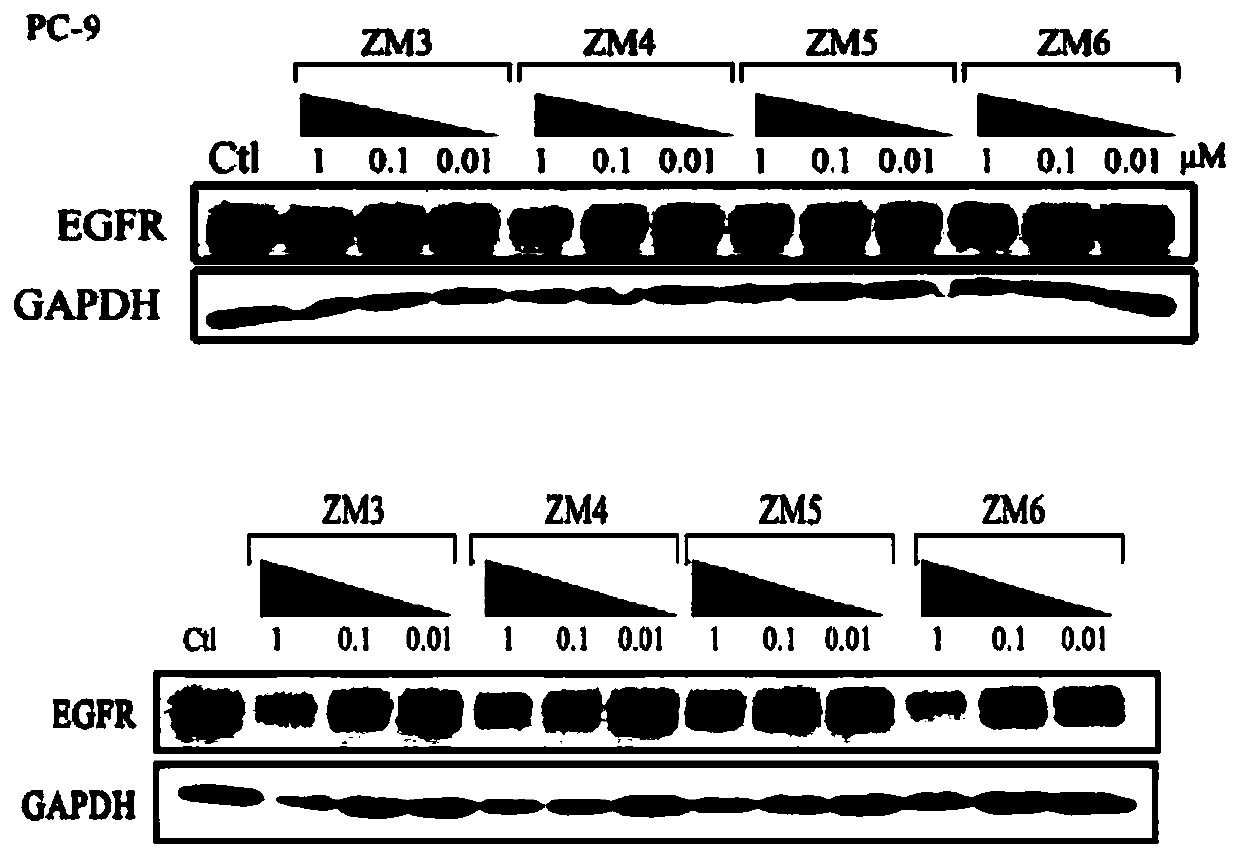
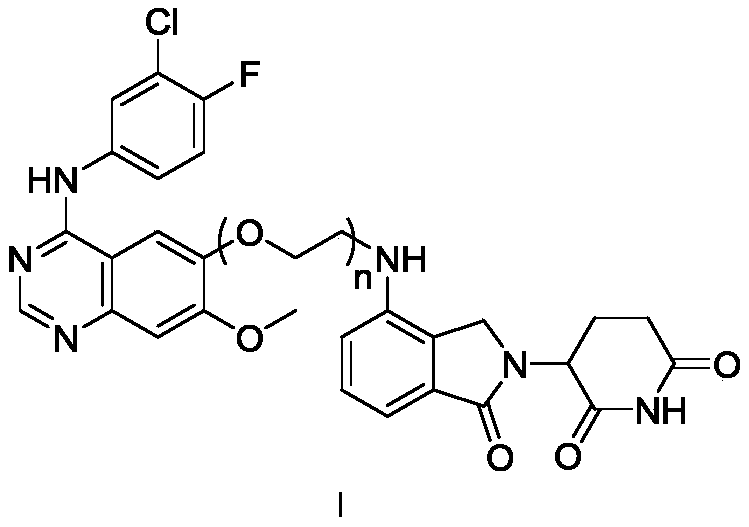
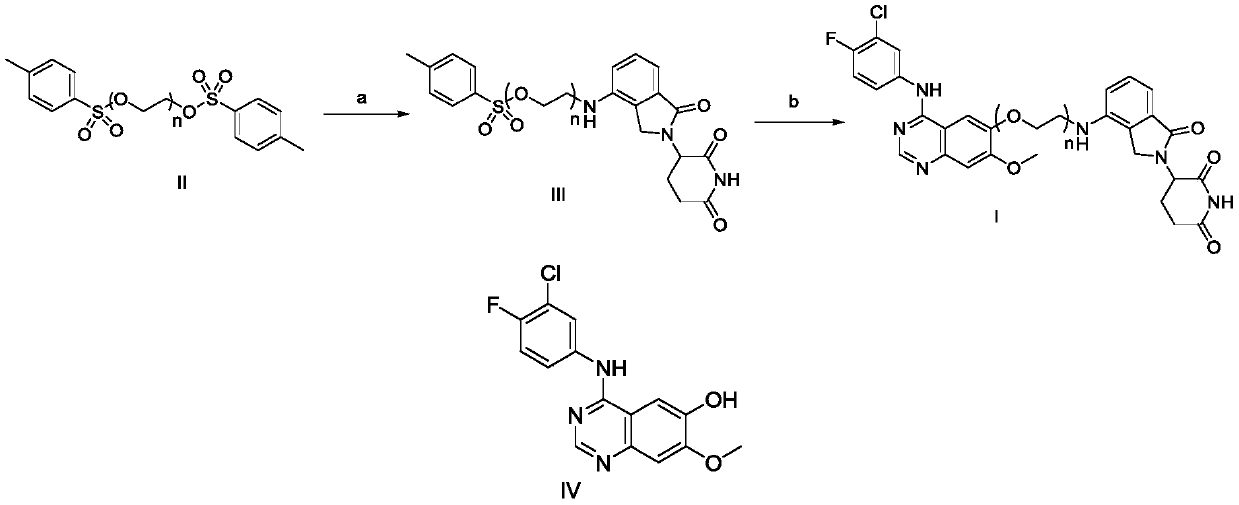
Compound based on CRBN ligand inducing EGFR degradation and preparation method thereof, pharmaceutical composition and application
A compound and composition technology, applied in the field of pharmaceutical compound synthesis, can solve the problems of side effects, reduce the anti-tumor effect of EGFR inhibitors, drug resistance, etc., and achieve the effects of reducing toxic side effects, excellent EGFR protein degradation, and good anti-tumor activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Preparation of 2-(2-((2-(2,6-piperidinedione-3-yl)-1-oxoindoline-4-yl)amino)ethoxy)ethyl-4 -Toluenesulfonate (2-1):
[0032]
[0033] Add diethylene glycol bis-p-toluenesulfonate (414mg, 1mmol), II-a (258mg, 1mmol), potassium carbonate (150mg, 1.2mmol) and DMF10mL in a 25mL three-necked flask, and react at 100°C under nitrogen protection After 2 hours, the reaction solution was poured into water, extracted with ethyl acetate, the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, and separated by medium chromatography to obtain 296 mg of a light yellow solid with a yield of 60%. 1 H NMR (400MHz, CDCl 3 )δ7.81(d, J=8.3Hz, 2H), 7.33(m, 4H), 6.92(d, J=7.2Hz, 1H), 5.17(m, 1H), 4.24(m, 2H), 4.16– 4.11(m,2H),4.03(t,J=5.7Hz,2H),3.69–3.64(m,2H),3.65(m,2H),3.03–2.75(m,2H),2.44(s,3H) ,2.23(m,2H).
[0034] (2) Add 2-1 (500mg, 1mmol), II-a (320mg, 1mmol), potassium carbonate (150mg, 1.2mmol) and DMF10mL to 25mL of three necks, ...
Embodiment 2
[0039] The specific preparation method is the same as in Example 1, and 3-(4-((2-(2-(2-((4-((4-fluoro-3-chlorophenyl) amino)-7-methoxyquin Azolin-6-yl)oxo)ethoxy)ethoxy)ethyl)amino)-1-oxoindolin-2-yl)piperidine-2,6-dione(1-2) , whose structure is as follows:
[0040]
[0041] 1 H NMR (400MHz, DMSO) δ9.62(s, 1H), 8.54(s, 1H), 8.13(dd, J=6.8, 2.6Hz, 1H), 7.81–7.73(m, 2H), 7.44(t, J=9.1Hz, 1H), 7.27–7.10(m, 2H), 6.97(d, J=7.1Hz, 1H), 6.74(d, J=7.4Hz, 1H), 5.15(m, 1H), 4.33– 4.17(m,3H),4.06(m,1H),3.94(s,3H),3.82(t,J=4.1Hz,2H),3.62(t,J=5.7,3.4Hz,2H),3.52-3.46 (m,8H),3.09–2.97(m,1H),2.84–2.75(m,1H),2.36–2.19(m,1H),2.03(m,1H).HRMS m / z: calcd for C 34 h 35 ClFN 6 o 7 [M+H] + 693.0414, found 693.0398.
Embodiment 3
[0043] The specific preparation method is the same as in Example 1, and 3-(4-((2-(2-(2-(2-((4-((4-fluoro-3-chlorophenyl) amino) amino)-7-methyl Oxyquinazolin-6-yl)oxo)ethoxy)ethoxy)ethoxy)ethyl)amino)-1-oxoindolin-2-yl)piperidine-2,6- Diketone (1-3), its structure is as follows:
[0044]
[0045] 1 H NMR (400MHz, DMSO) δ9.60(s, 1H), 8.52(s, 1H), 8.10(dd, J=6.8, 2.6Hz, 1H), 7.79–7.72(m, 2H), 7.41(t, J=9.1Hz, 1H), 7.24–7.06(m, 2H), 6.90(d, J=7.1Hz, 1H), 6.74(d, J=7.4Hz, 1H), 5.14(m, 1H), 4.30– 4.12(m,3H),4.01(m,1H),3.93(s,3H),3.79–3.70(m,4H),3.61(dd,J=5.7,3.4Hz,2H),3.50–3.42(m, 10H),3.07–2.93(m,1H),2.80–2.69(m,1H),2.34–2.17(m,1H),2.01(m,1H).HRMS m / z: calcd for C 36 h 39 ClFN 6 o 8 [M+H] + 737.0519, found 737.0522.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com