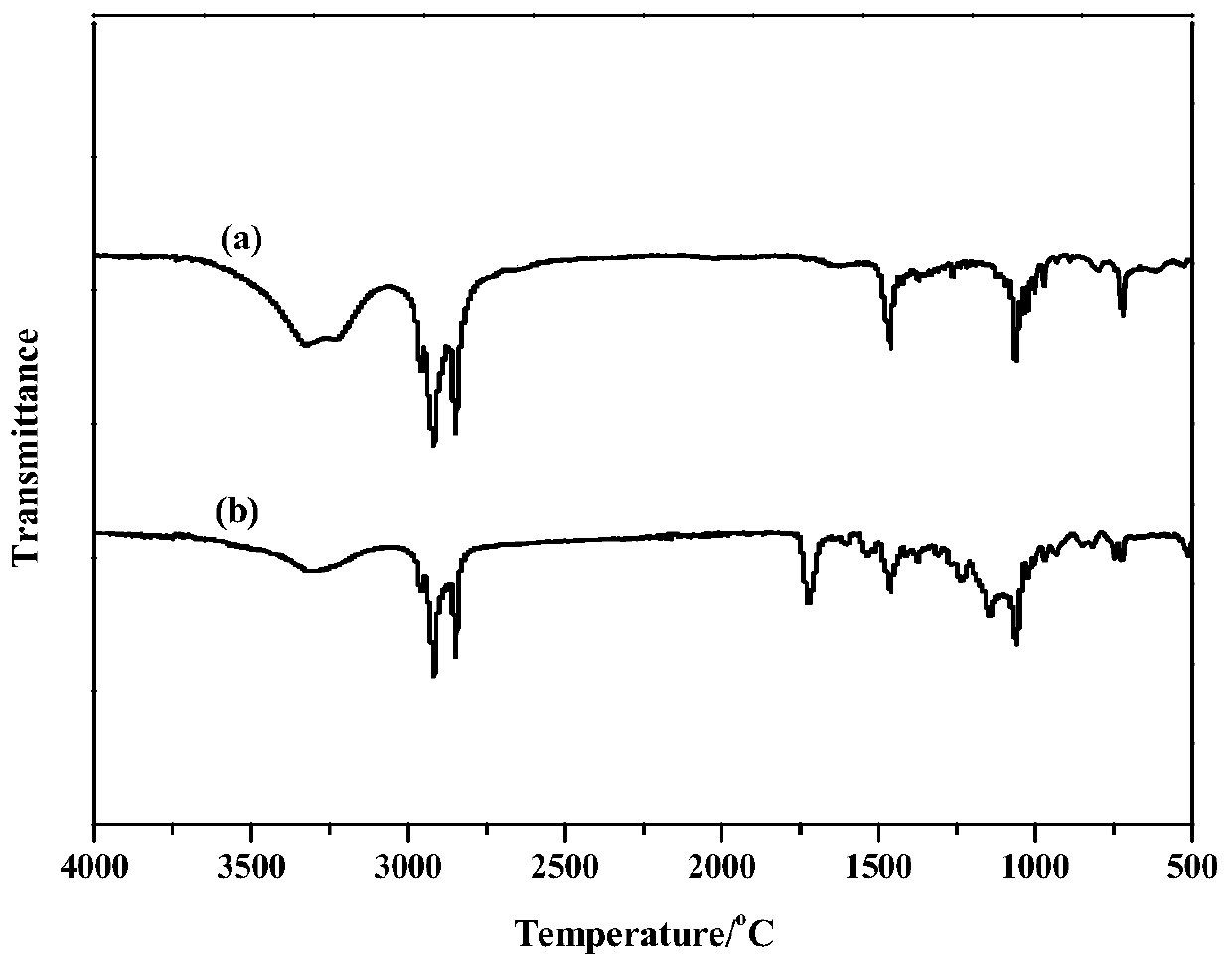
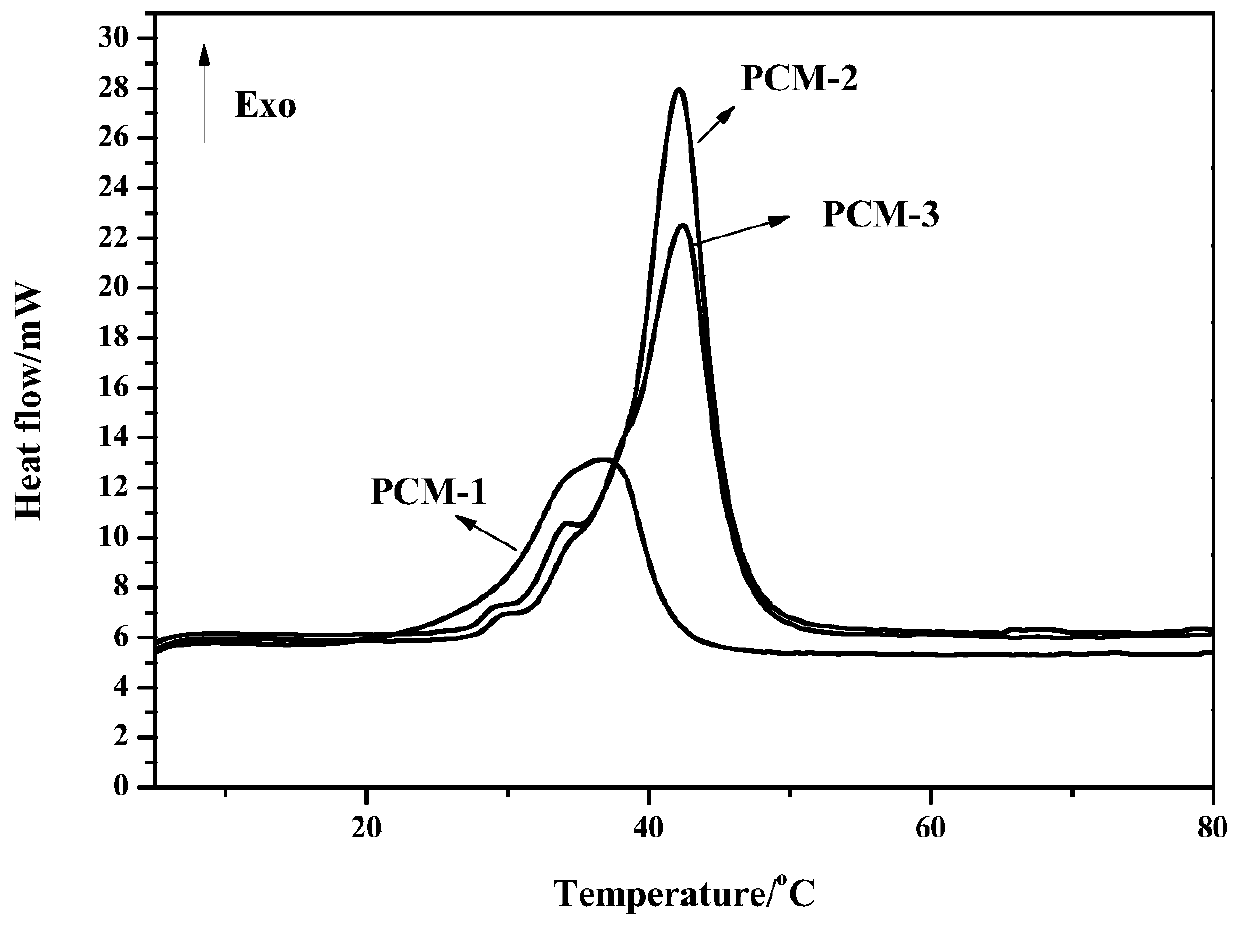
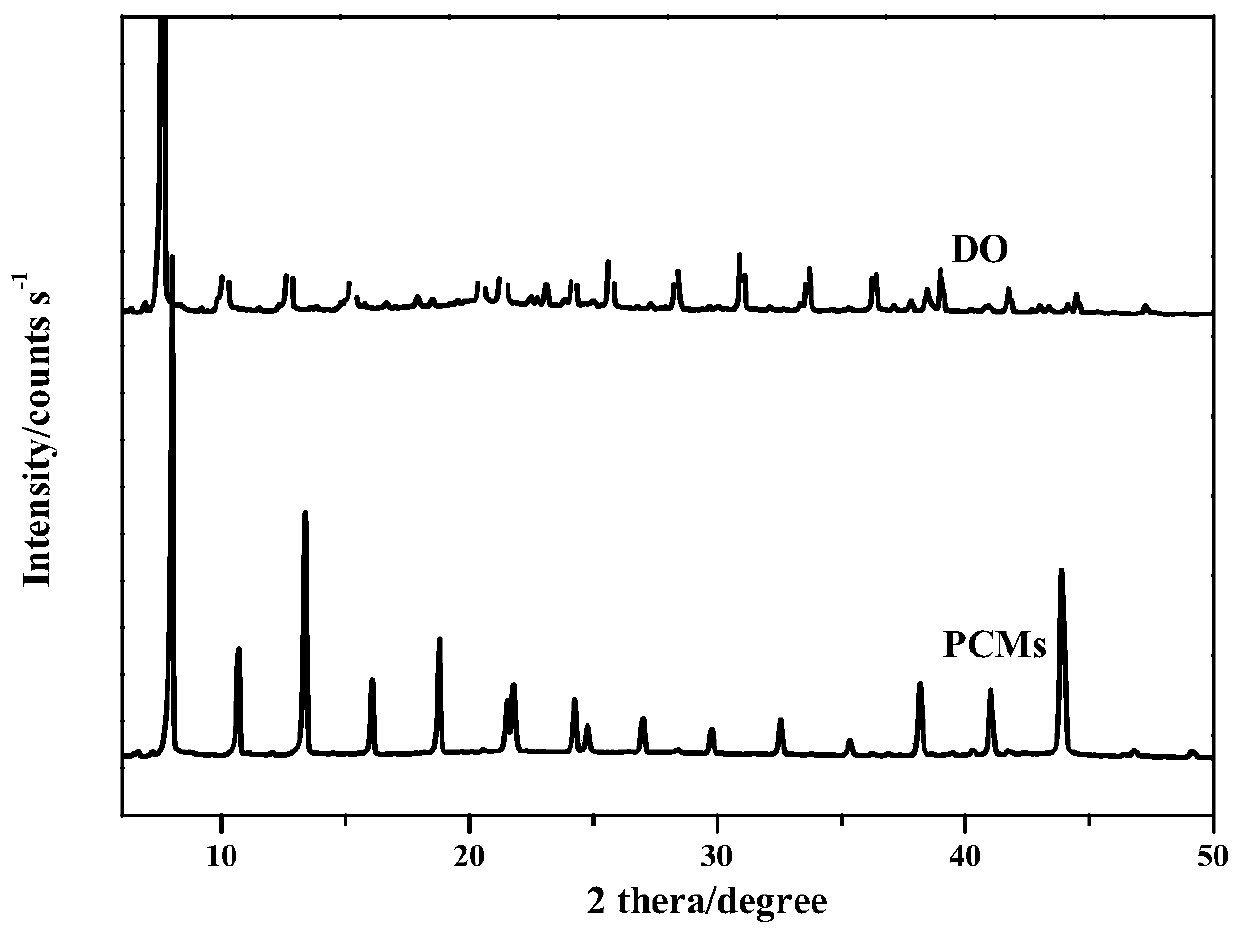
Room-temperature solid-solid phase change material for thermal energy storage and preparation method of room-temperature solid-solid phase change material
A technology of thermal energy storage and phase change materials, which is applied in the field of composite materials, can solve problems such as the influence of phase change enthalpy, the decline of crystallization ability, and the restriction of chain segment movement, and achieve the effect of low implementation cost and increased phase change enthalpy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Preparation of polyurethane dispersion
[0047] 5g (0.0025moL) polyether 220 (M n=2000) was vacuum-dried at 120°C for 4 hours by a circulating water pump, then lowered to 50°C, added 2.5g of diphenylmethane diisocyanate (0.01moL), reacted for 2h, then added 0.335g (0.0025moL) and dried in a vacuum oven at 80°C for 2h Dimethylolpropionic acid and 3 drops of dibutyltin dilaurate, heated to 75°C for 2 hours, cooled to 50°C, added 1.589g (0,012moL) hydroxyethyl methacrylate and 3 drops of dibutyltin dilaurate , and reacted for 2 hours to obtain a polyurethane prepolymer. During the process, due to the increase in viscosity, a total of 5g of acetone solution was added; when it was lowered to room temperature, 0.2525g of triethylamine was added for neutralization for 10min, and then 22g of deionized water was added to self-emulsify to obtain a double bond-containing Polyurethane dispersion;
[0048] (2) Preparation of polyurethane-acrylate emulsion
[0049] Take the ab...
Embodiment 2
[0053] (1) Preparation of polyurethane dispersion
[0054] 5g (0.005moL) polyether 220 (M n =2000) was vacuum-dried at 120°C for 4 hours by a circulating water pump, cooled to 50°C, added 2.5g of diphenyl diisocyanate (0.02moL), reacted for 2h, then added 0.82g (0.006moL) and dried in a vacuum oven at 80°C for 2h Dimethylolpropionic acid and 3 drops of dibutyltin dilaurate, react for 2h, add 2.918g hydroxyethyl methacrylate (0.022moL) and 3 drops of dibutyltin dilaurate, react for 2h, and obtain polyurethane prepolymer, process Due to the increase in viscosity, add a total of 5g of acetone solution; drop to room temperature and add 0.2525g of triethylamine to neutralize for 10min, then add 43g of deionized water to self-emulsify to obtain a polyurethane dispersion;
[0055] (2) Preparation of polyurethane-acrylate emulsion
[0056] Take the above polyurethane dispersion in a three-necked flask, add 1.25g methyl methacrylate (MMA), 0.625g butyl methacrylate (BMA), stir at a l...
Embodiment 3
[0060] (1) Preparation of polyurethane dispersion
[0061] 5g (0.0025moL) of polyether 220 and 0.335g (0.0025moL) of dimethylol propionic acid were dried in vacuum at 120°C for 4 hours, then lowered to 90°C, and 2.2g of isophorone diisocyanate (0.01moL) and 3 drops of diisocyanate were added. Dibutyltin laurate, react for 4h, cool down to 50°C, add 1.589g hydroxyethyl methacrylate (0.012moL) and 3 drops of dibutyltin dilaurate, react for 2h, and obtain polyurethane prepolymer, the viscosity increases during the process , add a total of 5g of toluene solution; drop to room temperature and add 0.2525g of triethylamine to neutralize for 10min, then add 22g of deionized water to self-emulsify to obtain a polyurethane dispersion;
[0062] (2) Preparation of polyurethane-acrylate emulsion
[0063] Take the above polyurethane dispersion in a three-necked flask, add 1.25g methyl methacrylate (MMA), 0.625g butyl methacrylate (BMA), stir at a low speed for 30min, then add 0.027g of AIB...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| phase transition enthalpy | aaaaa | aaaaa |
| solid content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com