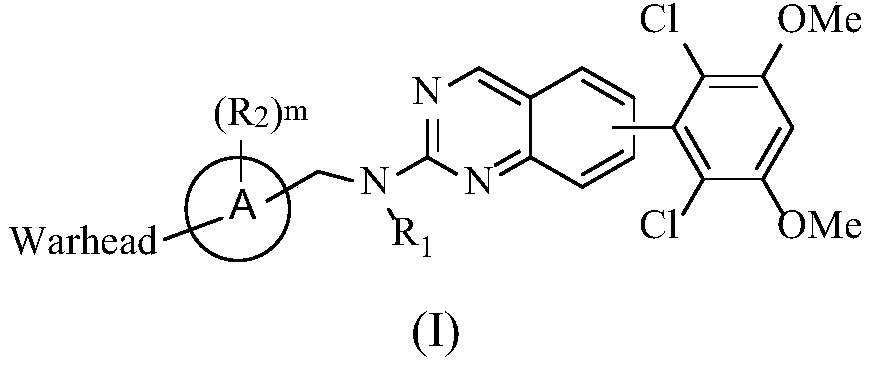
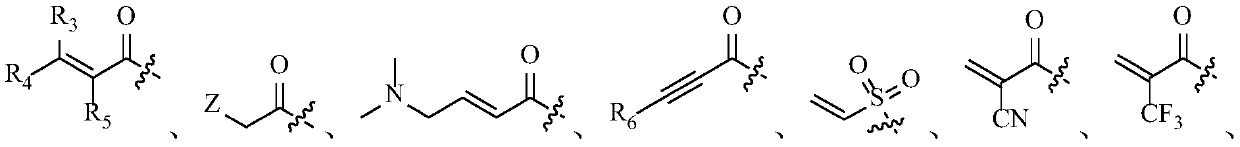
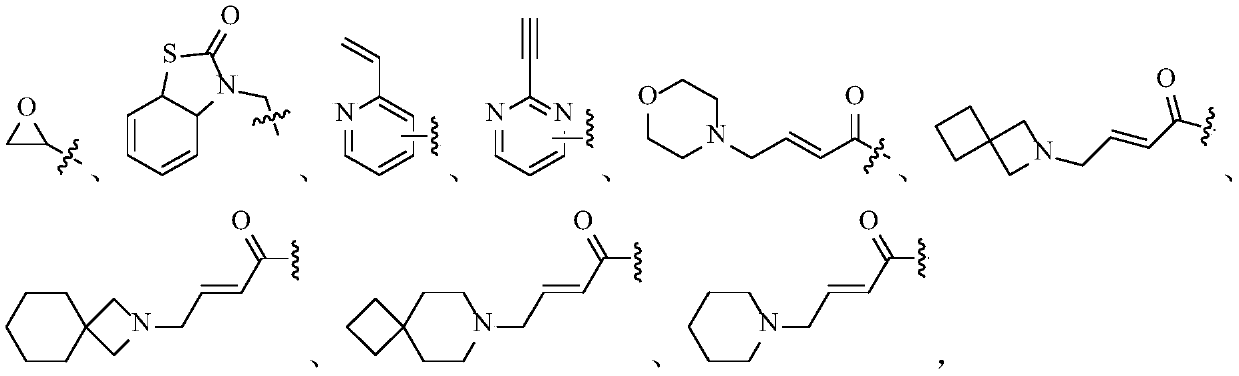
Fibroblast growth factor receptor antagonist compound
A growth factor receptor, fibroblast technology, applied in the field of medicine, can solve the problems of not strong and durable drug effect, easy to induce drug resistance, and not good selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0157] The described embodiments are only a part of the embodiments of the present invention, rather than all the embodiments. Based on the embodiments of the present invention, all other embodiments obtained by those of ordinary skill in the art without creative work shall fall within the protection scope of the present invention.
[0158] The abbreviation "NMP" used herein refers to N-methylpyrrolidone; "DIPEA" refers to N,N-diisopropylethylamine; "TLC" refers to thin layer chromatography; "PE:EA" refers to petroleum ether : Ethyl acetate; "TFA" means trifluoroacetic acid; "THF" means tetrahydrofuran; "EA" means ethyl acetate; "DCM:MeOH" means dichloromethane: methanol; "DCM" means dichloro Methane; "MTBE" refers to methyl tert-butyl ether; "TFAA" refers to trifluoroacetic anhydride.
Embodiment 1
[0159] Example 1: (R)-1-(3-(((6-(2,6-Dichloro-3,5-dimethoxyphenyl)quinazolin-2-yl)amino)methyl) Synthesis of pyrrolidin-1-yl)prop-2-en-1-one (compound 5)
[0160]
[0161] step:
[0162]
[0163] Synthesis of Intermediate I-1
[0164] Dissolve SM1 (5.00g, 20.5mmol) and SM2 (3.73g, 20.5mmol) in tetrahydrofuran (30ml), add cesium carbonate (20.00g, 61.5mmol) in water (30ml) solution, add a catalytic amount of Pd(PPh) 3 )Cl 2 , The resulting mixture was heated to reflux for 4 hours under a nitrogen atmosphere.
[0165] The reaction solution was concentrated to dryness and extracted with ethyl acetate. The organic phase was washed once with saturated sodium chloride, dried over anhydrous sodium sulfate, and the crude product obtained by concentration under reduced pressure was subjected to column chromatography (200-300 mesh silica gel, petroleum ether / ethyl acetate = 10 / 1) to obtain Intermediate I- 1 (3.80 g, 62% yield), is a pale yellow solid.
[0166] Synthesis of Intermediate I-2
[0...
experiment example 1
[0178] Experimental example 1: Cell activity test of the compound of the present invention
[0179] DMS114 is a small cell lung cancer FGFR abnormal cell
[0180] Test substance: the compound of the present invention, the structure of which is shown above.
[0181] Test equipment: use Espire multi-function microplate reader.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com