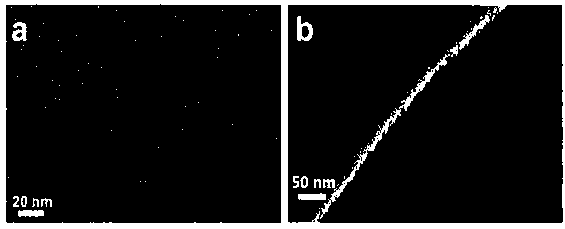
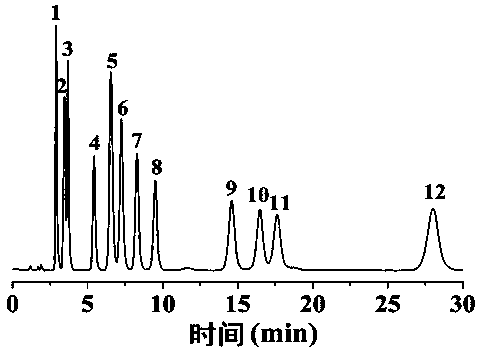
Preparation and application of tetraethylenepentamine carbon quantum dot/monomer co-bonded silica gel hydrophilic chromatography stationary phase
A tetraethylenepentamine carbon quantum and tetraethylenepentamine technology is applied in the field of separation of base nucleosides, amino acids and ginsenosides, achieving excellent separation effects, simple and reliable methods, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 5.0 g of tetraethylenepentamine was placed in a 100 mL three-necked flask, and then deoxygenated with nitrogen for 10 min. It was then heated to 240° C. and 1.0 g of anhydrous citric acid was added with vigorous stirring. After the reaction lasted for 1 min, the resulting reddish-brown solution was cooled to 190 °C, and then the reaction solution was blown with nitrogen to remove excess TEPA under continuous stirring until the total mass of the reaction solution became 2.0 g, and a mixture of TEPACDs and TEPA was obtained. Take 2.0g TEPACDs and TEPA, dissolve in 20 mL of anhydrous acetonitrile; take another 2.0 g gamma - Isocyanatopropyltriethoxysilane (ICPTES) was added to 10 mL of anhydrous acetonitrile, and the ICPTES solution was added dropwise to the mixed solution of TEPACDs and TEPA within 3 h under stirring (in the first Within hours, the reaction solution needs to be placed in ice water to keep the reaction at low temperature), after the ICPTES solution is add...
Embodiment 2
[0049] Take 5.0 g of tetraethylenepentamine in a 100 mL three-necked flask, and then deoxygenate it with nitrogen for 10 min; then heat it to 250 °C, add 2.0 g of anhydrous citric acid under vigorous stirring, and continue the reaction for 3 min. The reddish-brown solution was cooled to 160°C, and the reaction solution was blown with nitrogen to remove excess TEPA under continuous stirring until the total mass of the reaction solution became 3.0g to obtain a mixture of TEPACDs and TEPA; then 2.0g of TEPACDs and TEPA mixture was dissolved in 20 mL of anhydrous acetonitrile, then take 2.0 g gamma - Isocyanatopropyltriethoxysilane (ICPTES) was added to 10 mL of anhydrous acetonitrile, and the ICPTES solution was added dropwise to the mixed solution of TEPACDs and TEPA within 3 h under stirring (in the first Within hours, the reaction solution needs to be placed in ice water to keep the reaction at low temperature). After the addition of the ICPTES solution was completed, the rea...
Embodiment 3
[0051] Take 5.0 g of tetraethylenepentamine in a 100 mL three-necked flask, then heat it to 200 °C, add 2.0 g of anhydrous citric acid under vigorous stirring, continue the reaction for 5 min, and then cool the obtained red-brown solution to 150 °C. Then, the reaction solution was blown with nitrogen to remove excess TEPA under continuous stirring until the total mass of the reaction solution became 3.5 g, and a mixture of TEPACDs and TEPA was obtained. Dissolve 2.0 g of the above mixture in 20 mL of anhydrous acetonitrile, then add 4.0 g of γ-isocyanatopropyltriethoxysilane (ICPTES) into 10 mL of anhydrous acetonitrile, and dissolve the ICPTES solution within 3 h under stirring Add dropwise to the mixed solution of TEPACDs and TEPA (in the first hour of the reaction, the reaction solution needs to be placed in ice water to keep the reaction at low temperature). After the ICPTES solution was added dropwise, the reaction was continued for 11 h at room temperature. Finally, 2.0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com