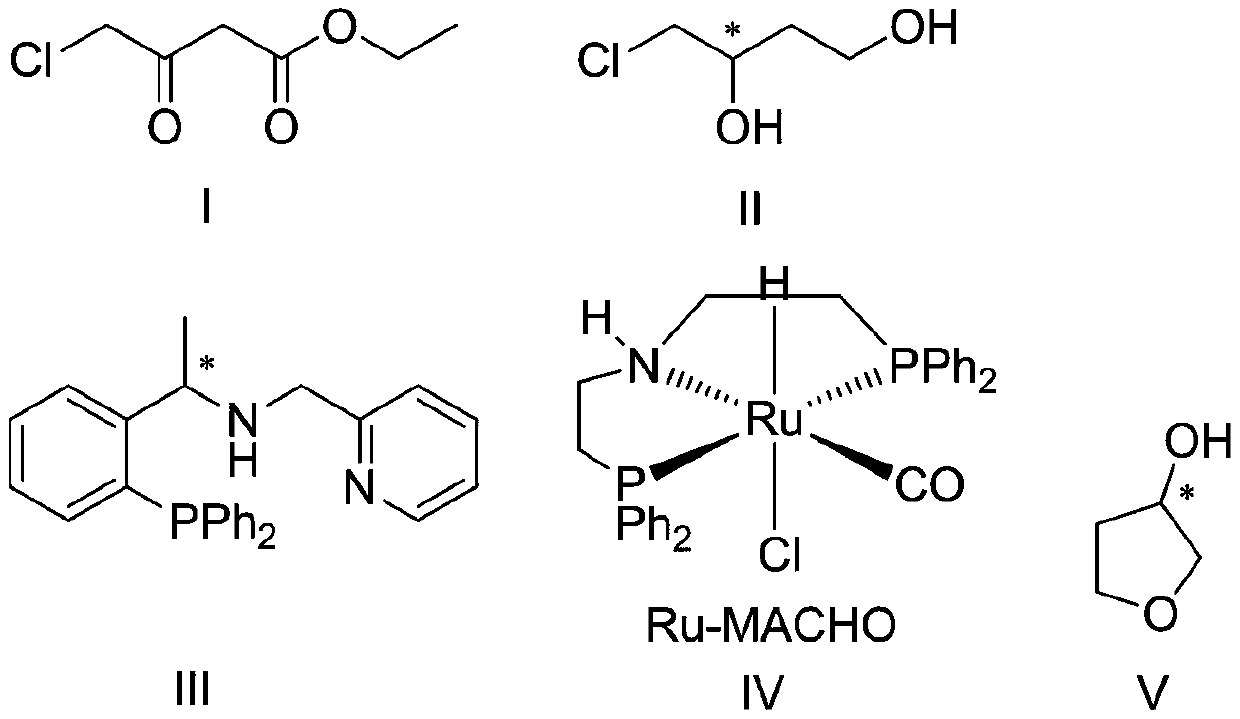
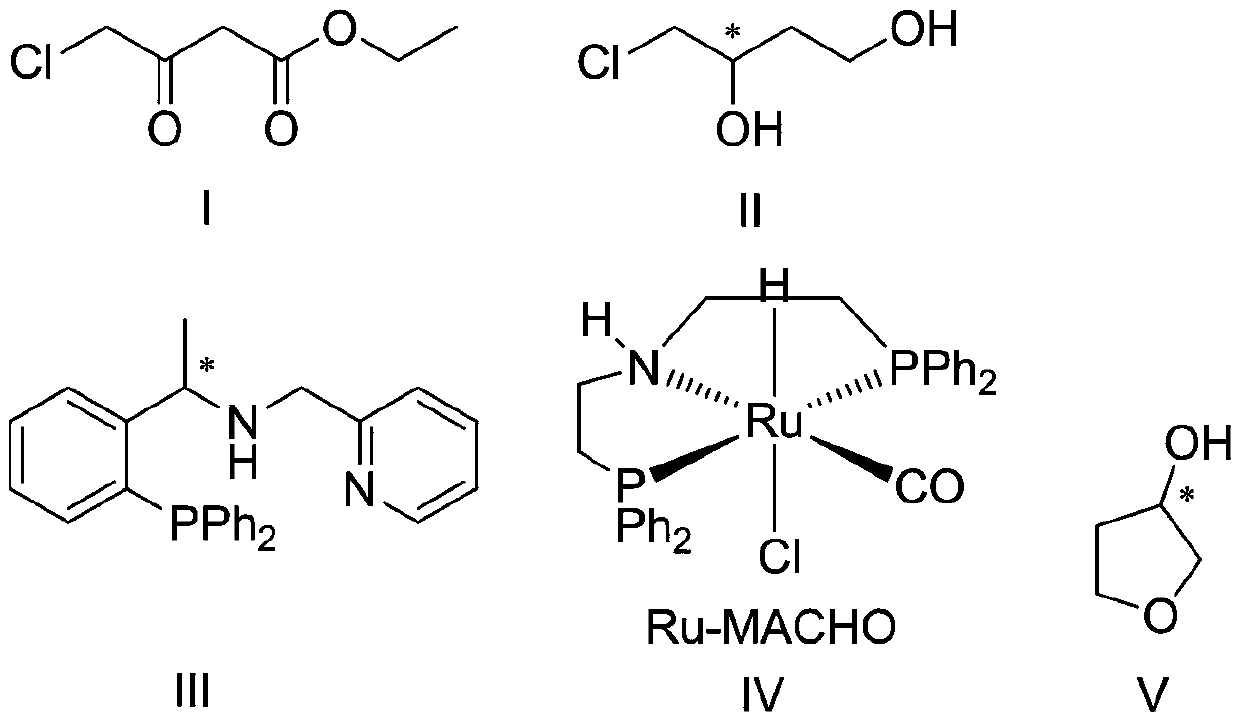
Preparation method of (s)-3-hydroxytetrahydrofuran
A technology of hydroxytetrahydrofuran and hydroxyl, which is applied in the field of preparation of -3-hydroxytetrahydrofuran, can solve the problems of unsatisfactory large-scale production, expensive biological enzymes, long reaction cycle, etc., and achieve low production cost, short reaction route and environmental pollution small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 1, preparation of (s)-4-chloro-3 hydroxy-1-butanol:
[0034] Step 1-1, under nitrogen protection, [Ir(COD)Cl] 2 1. The chiral phosphine-pyridine ligand was dissolved in ethanol and stirred at room temperature for 10 minutes to prepare the first catalyst.
[0035]Step 1-2, according to the amount of substance of the first catalyst theoretically prepared in step 1-1, according to the ratio of the amount of substance of the first catalyst and the amount of substance of ethyl 4-chloroacetoacetate to 1:3000, The substrate 4-chloroacetoacetate was dissolved in ethanol. Wherein, the volume ratio of the total volume of the ethanol used in step 1-1 and the ethanol used in step 1-2 to the substrate ethyl 4-chloroacetoacetate is 3:1. Then, the above ethanol solution of ethyl 4-chloroacetoacetate was added to the first catalyst prepared in step 1-1.
[0036] Step 1-3, according to the molar ratio of potassium tert-butoxide and ethyl 4-chloroacetoacetate is 1:15, the molar r...
Embodiment 2
[0040] Step 1, preparation of (s)-4-chloro-3 hydroxy-1-butanol:
[0041] Step 1-1, under nitrogen protection, [Ir(COD)Cl] 2 1. The chiral phosphine-pyridine ligand was dissolved in methanol and stirred at room temperature for 10 minutes to prepare the first catalyst.
[0042] Step 1-2, according to the amount of substance of the first catalyst theoretically prepared in step 1-1, according to the ratio of 1:2000 of the amount of substance of the first catalyst and the amount of substance of ethyl 4-chloroacetoacetate, The substrate 4-chloroacetoacetate was dissolved in methanol. Wherein, the volume ratio of the total volume of the methanol used in step 1-1 and the methanol used in step 1-2 to the substrate ethyl 4-chloroacetoacetate is 5:1. Then, the above methanol solution of ethyl 4-chloroacetoacetate was added to the first catalyst prepared in step 1-1.
[0043] Step 1-3, according to the molar ratio of sodium methylate and ethyl 4-chloroacetoacetate is 1:20, the molar ra...
Embodiment 3
[0047] Step 1, preparation of (s)-4-chloro-3 hydroxy-1-butanol:
[0048] Step 1-1, under nitrogen protection, [Ir(COD)Cl] 2 1. The chiral phosphine-pyridine ligand was dissolved in anhydrous dichloromethane and stirred at room temperature for 10 minutes to prepare the first catalyst.
[0049] Step 1-2, according to the amount of substance of the first catalyst theoretically prepared in step 1-1, according to the ratio of the amount of substance of the first catalyst and the amount of substance of ethyl 4-chloroacetoacetate to 1:5000, The substrate, ethyl 4-chloroacetoacetate, was dissolved in anhydrous dichloromethane. Wherein, the volume ratio of the total volume of the anhydrous dichloromethane used in step 1-1 and the anhydrous dichloromethane used in step 1-2 to the substrate ethyl 4-chloroacetoacetate is 2:1. Then, the above-mentioned ethyl 4-chloroacetoacetate solution in anhydrous dichloromethane was added to the first catalyst prepared in step 1-1.
[0050] Step 1-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com