Synthesis method of methyl 4-formyl-3-hydroxybenzoate
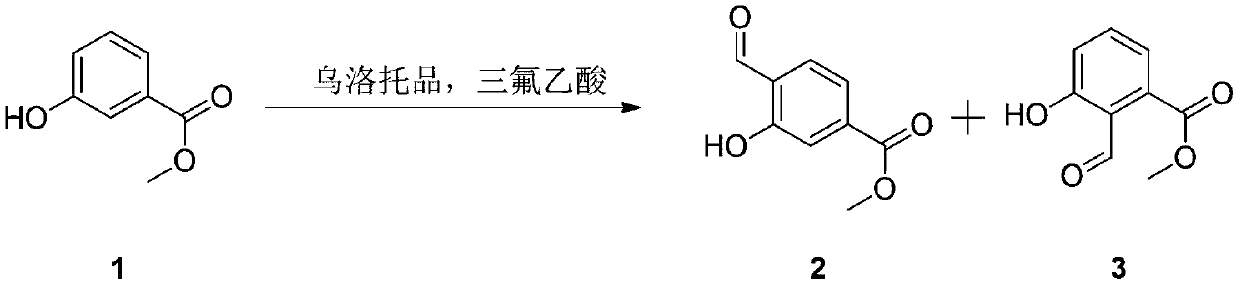
A technology for the synthesis of methyl hydroxybenzoate and its method, which is applied in the field of synthesis of methyl 4-formyl-3-hydroxybenzoate, and can solve the problems of difficult scale-up production of compounds, high cost of raw materials and catalysts, expensive palladium catalysts, etc. , to achieve the effect of good application prospects, good economic benefits, and simple routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
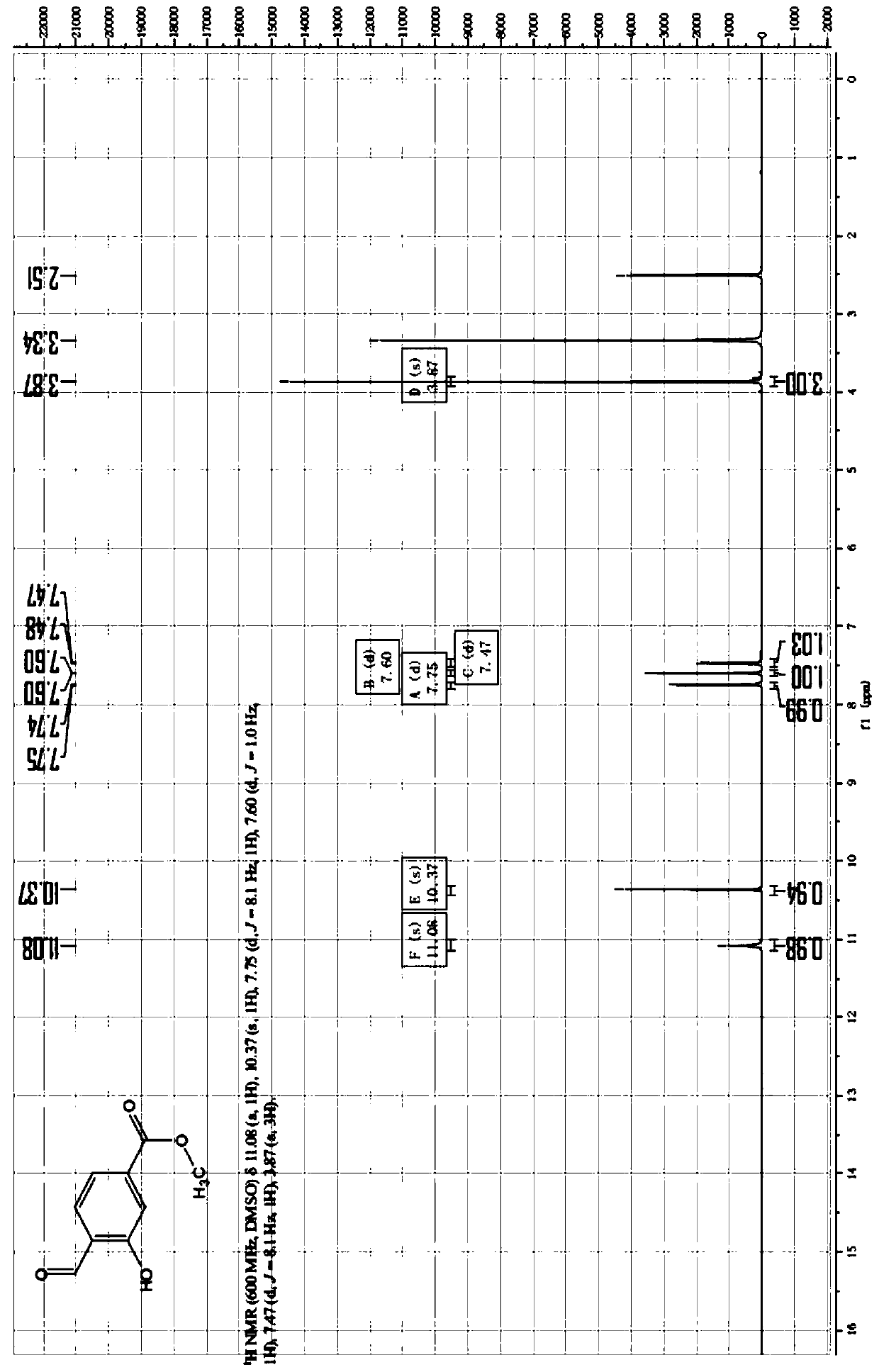
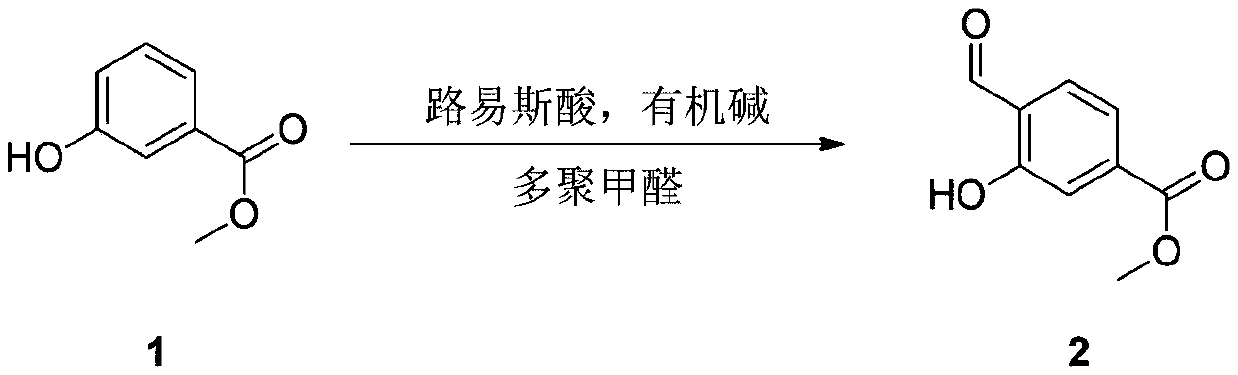
[0041] Under the protection of an inert gas, compound 1 (methyl 3-hydroxybenzoate) (300g, 1.97mol, 1eq) was added to acetonitrile (4.5L), and when the temperature was lowered to 0°C, slowly added in batches at this temperature 3 equivalents of anhydrous magnesium chloride (564g, 5.92mol, 3eq); after completion, continue to add triethylamine (995g, 9.85mol, 5eq) in batches to the reaction solution at this temperature; Paraformaldehyde (354g, 11.8mol, 6eq) was added in batches to the reaction solution, and after the addition was completed, the temperature was raised to 80°C, and the reaction was completed for 12 hours to obtain a reaction solution.
[0042] The reaction solution was lowered to room temperature, ethyl acetate (3 L) was added, and stirred for 0.5 hours. The reaction solution was poured into 3M aqueous hydrochloric acid (4 L), and the layers were separated. After layering, extract twice with ethyl acetate (1L*2), combine the organic phases, wash with w...
Embodiment 2
[0044] Under the protection of an inert gas, compound 1 (methyl 3-hydroxybenzoate) (152.0g, 1.0mol, 1eq) was added to acetonitrile (2.5L), and when the temperature was lowered to 0°C, slowly batchwise Add 3 equivalents of anhydrous zinc chloride (408.9g, 3.0mol, 3eq); after completion, continue to add triethylamine (506g, 5.0mol, 5eq) in batches to the reaction solution at this temperature; At this temperature, paraformaldehyde (180 g, 6.0 mol, 6 eq) was added in batches to the reaction solution. After the addition, the temperature was raised to 80° C., and the reaction was completed for 12 hours to obtain a reaction solution.
[0045] The reaction solution was lowered to room temperature, ethyl acetate (1.5 L) was added, and stirred for 0.5 hours. The reaction solution was poured into 3M aqueous hydrochloric acid (2L), and the layers were separated. After layering, extract twice with ethyl acetate (1L*2), combine the organic phases, wash with water, spin dry, beat with EA, a...
Embodiment 3
[0047] Under the protection of an inert gas, compound 1 (methyl 3-hydroxybenzoate) (152.0g, 1.0mol, 1eq) was added to acetonitrile (2.5L), and when the temperature was lowered to 0°C, slowly batchwise Add 3 equivalents of anhydrous magnesium chloride (285.6g, 3.0mol, 3eq); After completion, add paraformaldehyde (180g, 6.0mol, 6eq) in batches to the reaction solution at this temperature, raise the temperature to 80°C after the addition, and react for 12 hours to complete to obtain the reaction solution.
[0048] The reaction solution was lowered to room temperature, ethyl acetate (1.5 L) was added, and stirred for 0.5 hours. The reaction solution was poured into 3M aqueous hydrochloric acid (2L), and the layers were separated. After layering, extract twice with ethyl acetate (1L*2), combine the organic phases, wash with water, spin dry, beat with EA, and filter to obtain 135g of the product (yield: 75%, purity 97%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com