Preparation method of cyproheptadine hydrochloride
A technology of cyproheptadine hydrochloride and concentrated hydrochloric acid, applied in organic chemistry and other directions, can solve the problems of unfavorable employee health, unsuitability for industrialized large-scale production, and high energy consumption, reduce the use of organic solvents and hazardous chemicals, and is conducive to large-scale production. The effect of large-scale industrial production, production costs and production consumption reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
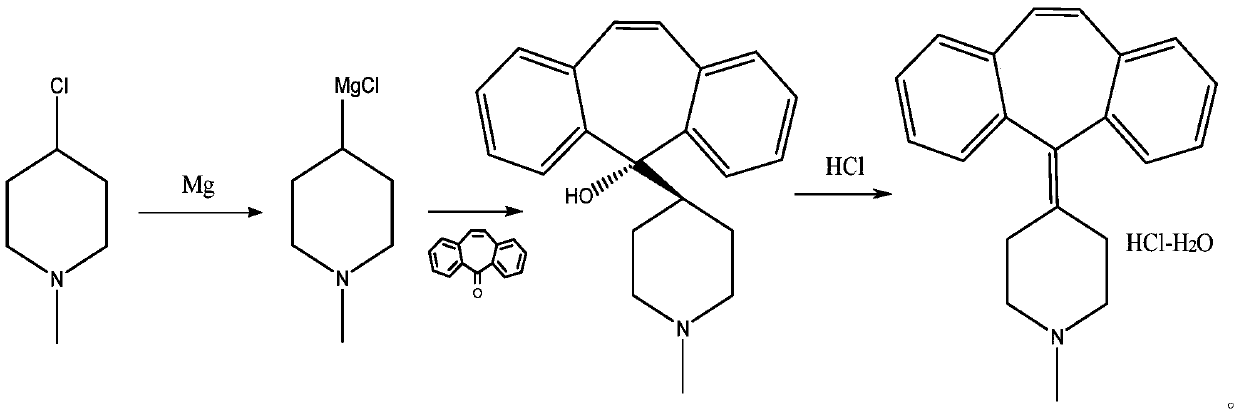
[0050] A preparation method of cyproheptadine hydrochloride, comprising the steps of:
[0051] 1) Add 0.89g of freshly polished magnesium bars to a 500mL reaction bottle, fill with nitrogen protection, add 50mL of tetrahydrofuran, heat to 60-65°C, then dropwise add 3.80g of 1-methyl-4-chloropiperidine to dissolve in 10mL For the solution of tetrahydrofuran, keep the reaction slightly boiling during the dropwise addition, and reflux for 1 hour after the dropwise addition, to obtain a 1-methyl-4-chloropiperidine magnesium solution;
[0052] 2) After cooling the 1-methyl-4-chloropiperidine magnesium solution to 0°C, slowly add 5.38g of dibenzo[a,e]cycloheptatrien-5-one, and react at room temperature for 1h to obtain 1- Methyl 4-(5-hydroxy-5-dibenzo[a,e]cycloheptatrienyl)piperidine solution;
[0053] 3) Distill the solution of 1-methyl 4-(5-hydroxy-5-dibenzo[a,e]cycloheptatrienyl)piperidine to tetrahydrofuran under reduced pressure at 40~50℃, then stir and cool to 0°C, slowly add ...
Embodiment 2
[0057] A preparation method of cyproheptadine hydrochloride, comprising the steps of:
[0058] 1) Add 9.0g of newly polished magnesium chips into a 2L reaction flask, fill with nitrogen protection, add 350mL of tetrahydrofuran, heat to 60-65°C, then add 300mL of 38g of 1-methyl-4-chloropiperidine in tetrahydrofuran , keep the reaction slightly boiling during the dropwise addition, and reflux for 1 hour after the dropwise addition, to obtain a 1-methyl-4-chloropiperidine magnesium solution;
[0059] 2) After cooling the 1-methyl-4-chloropiperidine magnesium solution to 5°C, slowly add 52g of dibenzo[a,e]cycloheptatrien-5-one, and react at room temperature for 1h to obtain 1- Methyl 4-(5-hydroxy-5-dibenzo[a,e]cycloheptatrienyl)piperidine solution;
[0060] 3) Distill 1-methyl 4-(5-hydroxy-5-dibenzo[a,e]cycloheptatrienyl)piperidine solution to tetrahydrofuran under reduced pressure at 40~50℃, then stir and cool to 4℃ , slowly add 1000mL distilled water, the speed of adding dist...
Embodiment 3
[0064] A preparation method of cyproheptadine hydrochloride, comprising the steps of:
[0065] 1) Add 1.96kg of newly polished magnesium chips into a 300L reaction bottle, fill it with nitrogen protection, add 30kg of tetrahydrofuran, heat to 60-65°C, and then add tetrahydrofuran containing 7.5kg of 1-methyl-4-chloropiperidine The solution was 70kg, and the reaction was kept slightly boiling during the dropwise addition. After the dropwise addition, the reaction was refluxed for 1h to obtain a 1-methyl-4-chloropiperidine magnesium solution;
[0066] 2) After cooling the 1-methyl-4-chloropiperidine magnesium solution to 5°C, slowly add 10.2 kg of dibenzo[a,e]cycloheptatrien-5-one, and react at room temperature for 1 hour to obtain 1- Methyl 4-(5-hydroxy-5-dibenzo[a,e]cycloheptatrienyl)piperidine solution;
[0067] 3) Distill 1-methyl 4-(5-hydroxy-5-dibenzo[a,e]cycloheptatrienyl)piperidine solution at 40~50℃ to remove tetrahydrofuran under reduced pressure, then stir and cool t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com