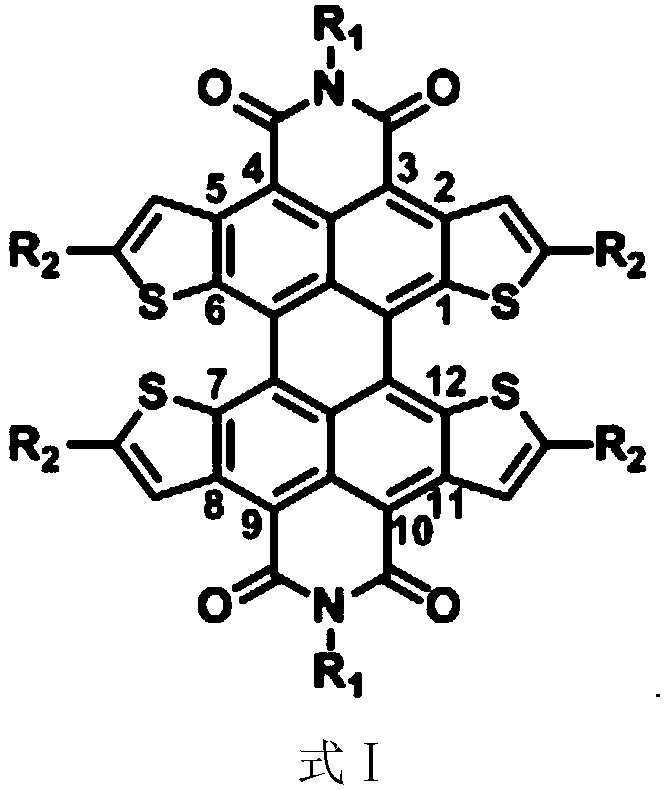
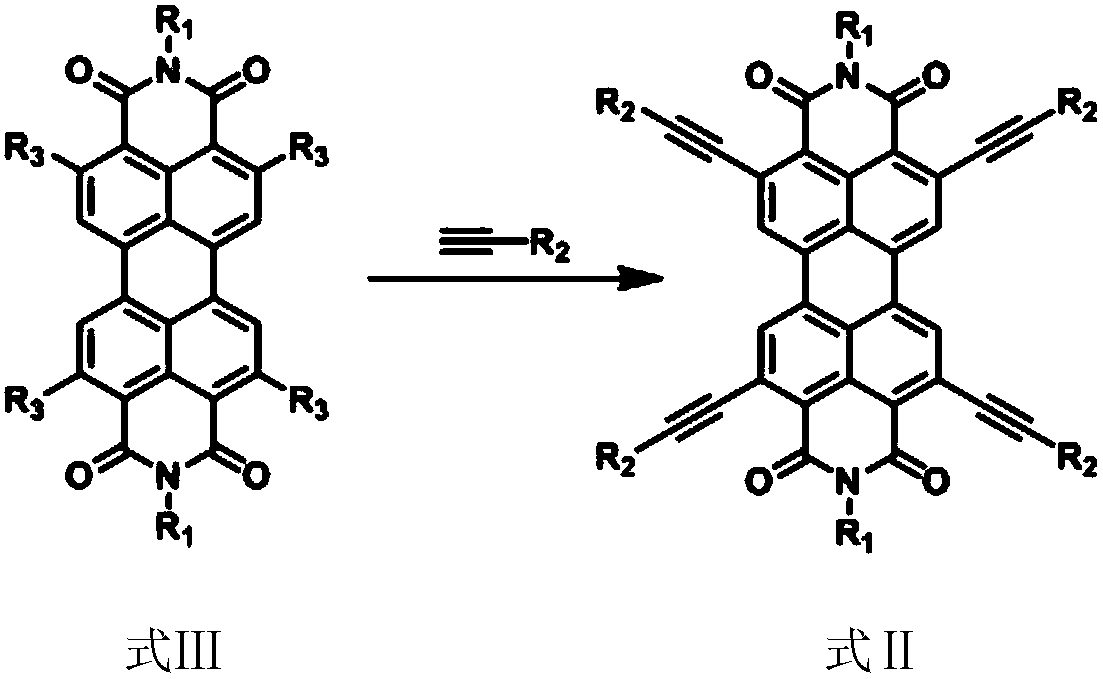
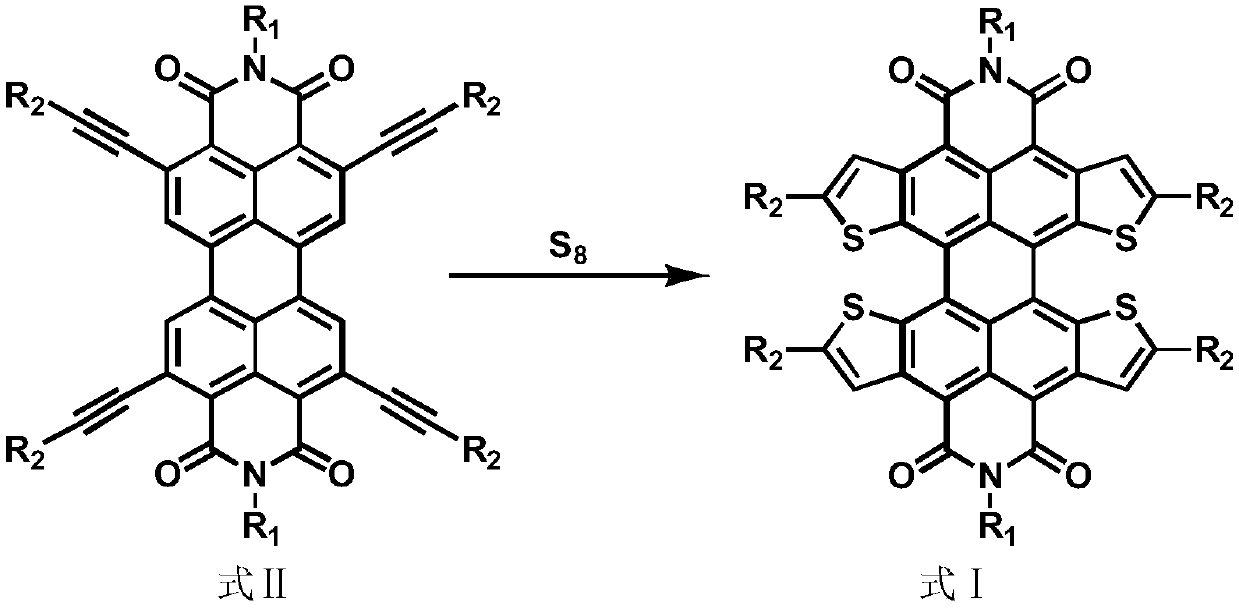
Quaterthiophene condensed perylene bisimide derivative and preparation method thereof
A technology of tetrathiophene-fused peryleneimide and thiophene-fused peryleneimide, which is applied in the field of tetrathiophene-fused peryleneimide derivatives and their preparation, and there are few derivatives that can solve nonbay position functionalization Reporting and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1, [1,2-b:6,5-b':7,8-b":12,11-b"']tetrathiophene fused peryleneimide derivative C 5,5 -Preparation of PTTI compound (formula Ⅰ-1)
[0101] 1) Preparation of 2,5,8,11-position tetraboronic acid ester substituted perylene imide derivatives (formula IV-1)
[0102]
[0103] Concrete synthetic steps:
[0104] In a 100ml sealed tube, add PDI (500mg, 0.715mmol) of formula Ⅴ-1, pinacol borate (1.45g, 5.723mmol), [Ir(OMe)cod] 2 (14mg, 0.02mmol), tris(pentafluorophenyl)phosphine (47mg, 0.09mmol), vacuumize and fill with argon, add 10ml of dioxane, heat to 110°C for 48h, cool the reaction to room temperature, spin to dry the solvent , silica gel column chromatography (CH 2 Cl 2 ), via CH 2 Cl 2 / MeOH recrystallization to obtain orange-yellow compound formula IV-1 (671 mg, 78%).
[0105] 1 H NMR (CDCl 3 ):δ=0.80(t,12H),1.21(m,32H),1.59(s,48H),1.73-1.78(m,4H),2.09-2.14(m,4H),5.01-4.94(m,2H ), and 8.44(s,4H)ppm; HRMS(MALDI(N),100%):calcd(%)for C 70 h 98 B 4 N ...
Embodiment 2
[0125] Example 2, [1,2-b:6,5-b':7,8-b":12,11-b"']tetrathiophene fused peryleneimide derivative C4F-PTTI compound ( The synthetic route of formula Ⅰ-2):
[0126] 1) Preparation of 2,5,8,11-position tetraboronic acid ester substituted perylene imide derivatives (formula IV-2)
[0127] The reaction equation is as follows, and the specific steps are referring to step 1) of Example 1.
[0128]
[0129]2) Preparation of 2,5,8,11-tetrabromo-substituted perylene imide derivatives (formula III-2)
[0130] The reaction equation is as follows, and the specific steps are referring to step 2) of Example 1.
[0131]
[0132] 3) Preparation of 2,5,8,11-tetrakynyl substituted perylene imide derivatives (Formula II-2)
[0133] The reaction equation is as follows, and the specific steps are referring to step 3 of Example 1).
[0134]
[0135] Characterization parameters:
[0136] 1 H NMR (400MHz, CDCl 3 ): δ=8.60(s, 4H), 5.09-5.02(m, 4H), 1.25-1.24(d, J=4.0Hz, 84H); 13 C NMR (1...
Embodiment 3
[0147] Example 3, [1,2-b:6,5-b':7,8-b":12,11-b"'] tetrathiophene fused perylene imide derivative C 5,5 -Preparation of PTTI-Ph compound (formula Ⅰ-3)
[0148] 1) Preparation of 2,5,8,11-tetraphenylethynyl substituted perylene imide derivatives (Formula II-3)
[0149]
[0150] Concrete synthetic steps:
[0151] Add compound formula III-1 (500mg, 0.493mmol), phenylacetylene (503mg, 4.928mmol), Pd (pph 3 ) 2 Cl 2 (70mg, 0.1mmol), evacuated and filled with argon, added 20ml THF, heated to 40°C for 2h, cooled to room temperature, poured into 500ml water, filtered with suction, dried, and separated by silica gel column chromatography DCM:PE=1: 4. Obtain compound formula II-3 (440 mg, 80%). HRMS(MALDI(N),100%): calcd(%) for C 40 h 70 N 2 o 4 :1250.248; found, 1250.247.
[0152] 2) [1,2-b:6,5-b':7,8-b":12,11-b"'] tetrathiophene fused perylene imide derivatives (formula Ⅰ-3) preparation
[0153] The reaction equation is as follows, and the specific steps are referring to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com