Tetraphenylethylene-containing organic semiconductor material, and preparation method and application thereof
An organic semiconductor, tetraphenylethylene technology, applied in the field of organic photoelectric materials, can solve the problems of non-luminescence and unfavorable fluorescence emission, etc., and achieve the effect of simple storage, excellent photoelectric performance and stable material structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
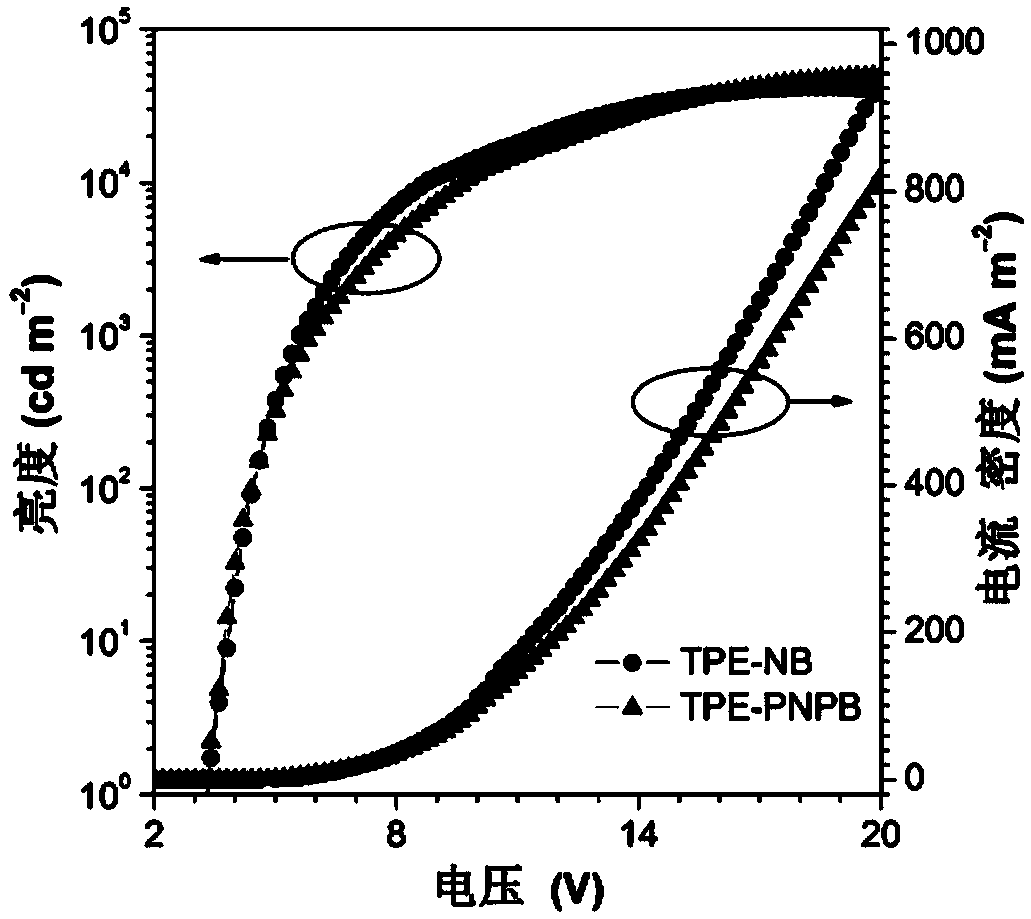
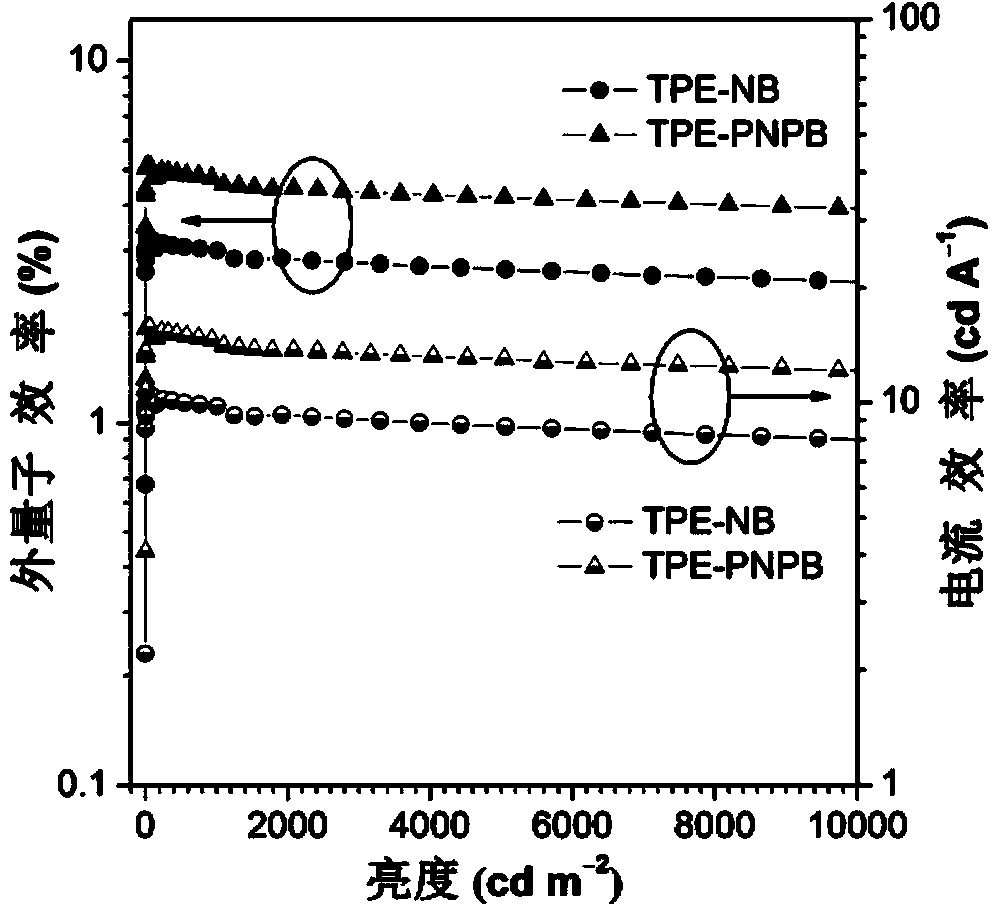
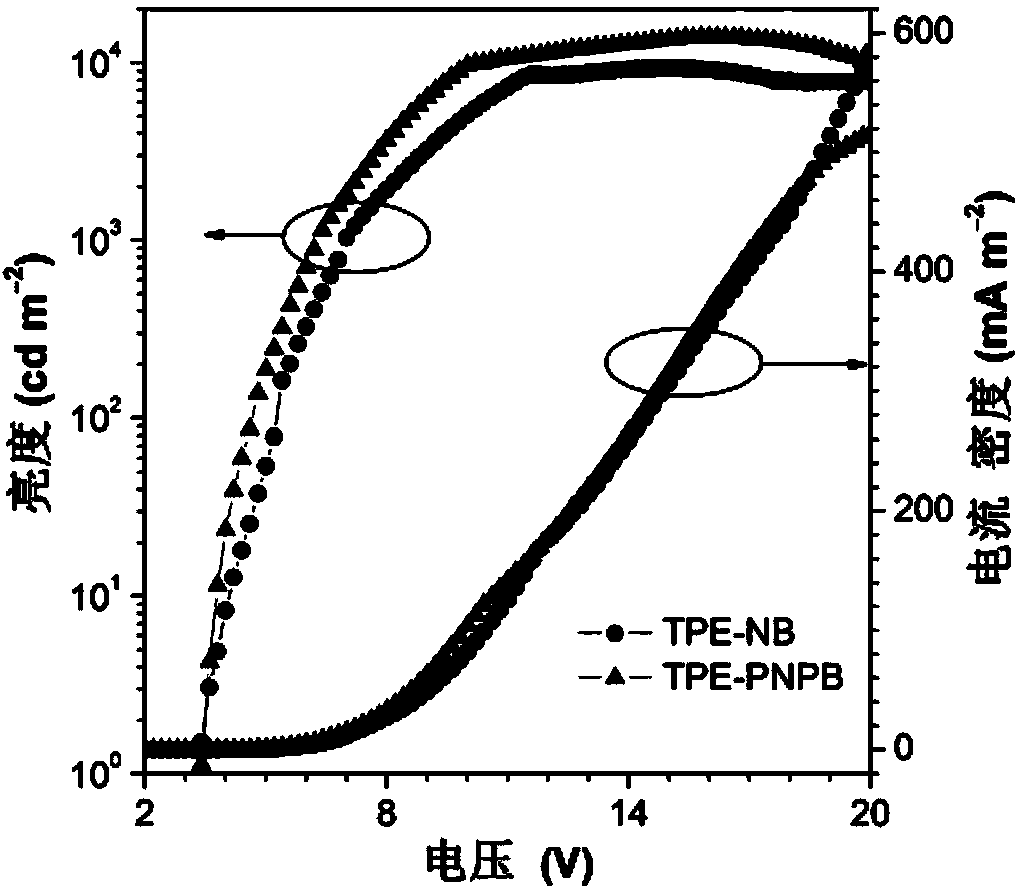
Image
Examples
Embodiment 1
[0037] Embodiment 1: Preparation of organic semiconductor material (TPE-NB) containing tetraphenylethylene
[0038]
[0039] Reaction equation (1):
[0040]
[0041](1) Intermediate 1 was prepared according to the method disclosed in the literature (J. Am. Chem. Soc. 1954, 76, 3502). The raw materials 4-bromobenzophenone and dimethyl boron fluoride were ordered directly from TCI Corporation.
[0042] (2) 4-bromobenzophenone (3.92g, 15mmol), intermediate 1 (1.75g, 5mmol) and zinc powder (1.30g, 20mmol) were added to the reaction flask, and the gas was changed three times. Inject THF (80mL) down, cool to -78°C, add TiCl dropwise 4 (1.90g, 10mmol), after the dropwise addition was completed, the reaction system was returned to room temperature, and heated under reflux at 70°C for 12h. Saturated sodium carbonate solution was added until a large amount of solids were precipitated, filtered, the filtrate was extracted with dichloromethane, concentrated and powdered, and wash...
Embodiment 2
[0044] Embodiment 2: Preparation of organic semiconductor material (TPE-PNPB) containing tetraphenylethylene
[0045]
[0046] Reaction equation (2):
[0047]
[0048] (1) Intermediate 3 was prepared according to the method disclosed in the literature (Chem.Commun.2011, 47, 6924); Intermediate 5 was prepared according to the method disclosed in the literature (Chem.Eur.J.2014, 10, 994). 4-Bromobenzophenone was ordered directly from TCI Corporation.
[0049] (2) Add 4-bromobenzophenone (3.92g, 15mmol), intermediate 3 (2.06g, 5mmol) and zinc powder (1.30g, 20mmol) into the reaction flask, pump and change gas three times, and Inject THF (80mL) down, cool to -78°C, add TiCl dropwise 4 (1.90g, 10mmol), after the dropwise addition was completed, the reaction system was returned to room temperature, and then heated under reflux at 80°C for 12h. Saturated sodium carbonate solution was added until a large amount of solids were precipitated, filtered, the filtrate was extracted...
Embodiment 3
[0052] Embodiment 3: Preparation of organic semiconductor material (TPE-DB) containing tetraphenylethylene
[0053]
[0054] Reaction equation (3):
[0055]
[0056] (1) Intermediate 6 (4,4'-dibromotetraphenylethylene) was prepared according to the method disclosed in the literature (J. Mater. Chem. 2012, 22, 232).
[0057] (2) Intermediate 6 (980mg, 2mmol) was added to the reaction flask, pumped and ventilated three times, THF (60mL) was injected under nitrogen protection, cooled to -78°C, and n-BuLi (1.6M, 2.8 mL, 4.4mmol), reacted at this temperature for 2 hours, dissolved dimethyl boron fluoride (1.18g, 4.4mmol) in THF (20mL) and added to the reaction system, and continued to react at -78°C for 1 hours, then returned to room temperature and stirred overnight. After concentrating, it was made into a powder, and was passed through a column with an eluent (petroleum ether / dichloromethane=10 / 1) to obtain the final product TPE-DB with a yield of 90%.
[0058] 1 H NMR ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com