Solution-processable two-dimensional conjugated organic molecule photovoltaic material, preparation method and application thereof
A reaction and carbon atom technology, applied in photovoltaic power generation, organic chemistry, semiconductor/solid-state device manufacturing, etc., can solve problems such as low hole mobility, low filling factor of photovoltaic devices, weak interaction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, the synthesis of α-Bromine β-hexylthienylvinindanone (in the formula III, R 3 =C 6 h 13, n=1 compound)
[0034] With 3.03g of indanone (formula IV), 6.25g of compound (formula V, n=1, R 3 =C 6 h 13 ), 2ml of piperidine and 22.8ml of dried acetonitrile were added to the reactor for mixing and dissolving, and then refluxed at 80° C. for 24 hours under the protection of nitrogen. The reaction mixture was cooled to room temperature, then dissolved in dichloromethane, washed with brine, dried over anhydrous magnesium sulfate, evaporated the solvent in vacuo, and washed with dichloromethane / petroleum ether (1 / 5, V / V) Purified by chromatographic column to obtain 5 g of yellow solid α-Bromine β-hexylthienylvinindanone, yield: 60%. GC / MS: 402 (M+).
[0035] The structural confirmation data are as follows: 1 H NMR (400MHz, CDCl 3 )δ (ppm): 7.96-7.95 (t, 2H), 7.835 (s, 1H), 7.81-7.77 (m, 2H), 7.599 (s, 1H), 2.64-2.60 (t, 2H), 1.66-1.58 (m, 3H), 1.38-1.25 (m...
Embodiment 2
[0036] Embodiment 2, the synthesis of α-Bromine β-hexyl dithienylvinindanone (in the formula III, R 3 =C 6 h 13 , n=2 compounds)
[0037] With 1.34g of indanone (formula IV), 4.0g of compound (formula V, n=2, R 3 =C 6 h 13 ), 2ml of piperidine and 22.8ml of dried acetonitrile were added to the reactor for mixing and dissolving, and then refluxed at 80° C. for 24 hours under the protection of nitrogen. The reaction mixture was cooled to room temperature, then dissolved in dichloromethane, washed with brine, dried over anhydrous magnesium sulfate, evaporated the solvent in vacuo, and washed with dichloromethane / petroleum ether (1 / 5, V / V) Purified by chromatographic column to obtain 5 g of yellow solid α-Bromine β-hexyldithiophene vinylindanone, yield: 60%. MALDI-TOF: MS: 569.1, calculated for C 30 h 33 BrO 2 S 2 The mass-to-charge ratio is 569.62.
[0038] The structural confirmation data are as follows: 1 H NMR (400MHz, CDCl 3 )δ (ppm): 8.09-8.07 (s, 1H), 7.97-7.94...
Embodiment 3
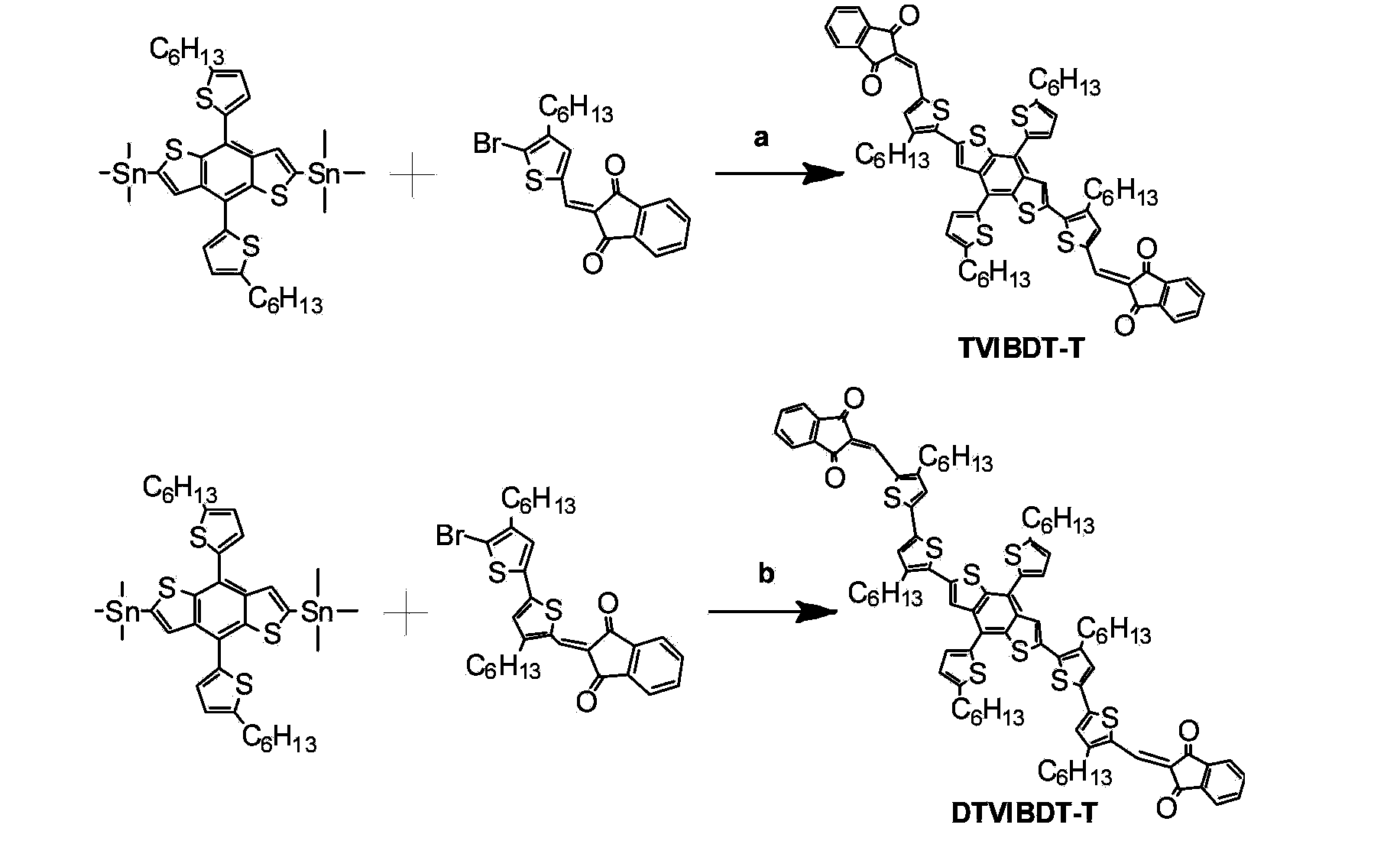
[0039] Embodiment 3,2,6-dithiophene vinyl indanone 4,8-dithiophene benzo [1,2-b:4,5-b'] synthesis of dithiophene (TVIBDT-T) (in the formula I, R 1 = R 3 =C 6 h 13 , R 2 =H, the compound of n=1)
[0040] Add 0.523g monomer formula II, 0.5g monomer α-Bromine β-hexylthienylvinindanone and 20mL toluene to a 50mL three-necked flask, and after 15 minutes of passing nitrogen, add 30mg tetrakis(triphenylphosphine) palladium ( 0.026mmol), the temperature was raised to 115°C and the reaction was stirred for 24 hours. After the reaction was completed, it was cooled to room temperature, and the reaction solution was settled in 200 mL of methanol, and then filtered with suction to collect a black solid. Then adopt dichloromethane / petroleum ether (1 / 1, v / v) to separate and purify the product through a chromatographic column to obtain 0.575 g of black molecular TVIBDT-T solid, with a yield of 80%. MALDI-TOF: m / z 1166.6. Calculated C 70 h 70 o 4 S 6 The mass-to-charge ratio is 1166....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com