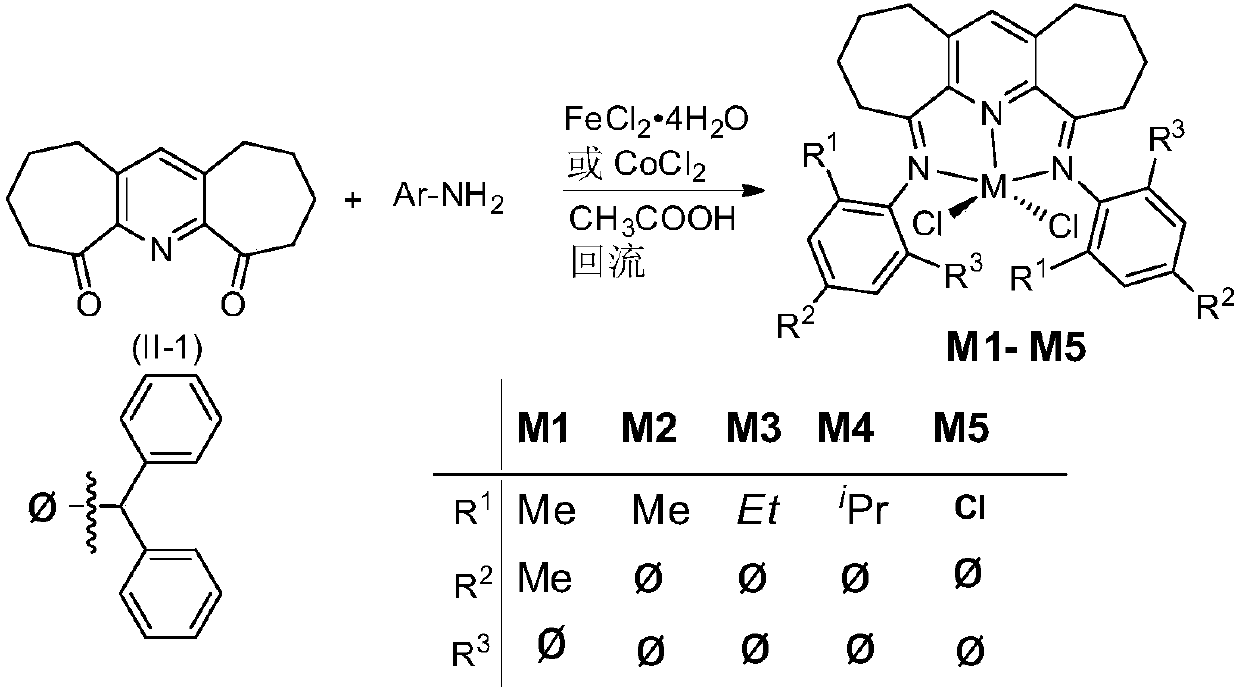
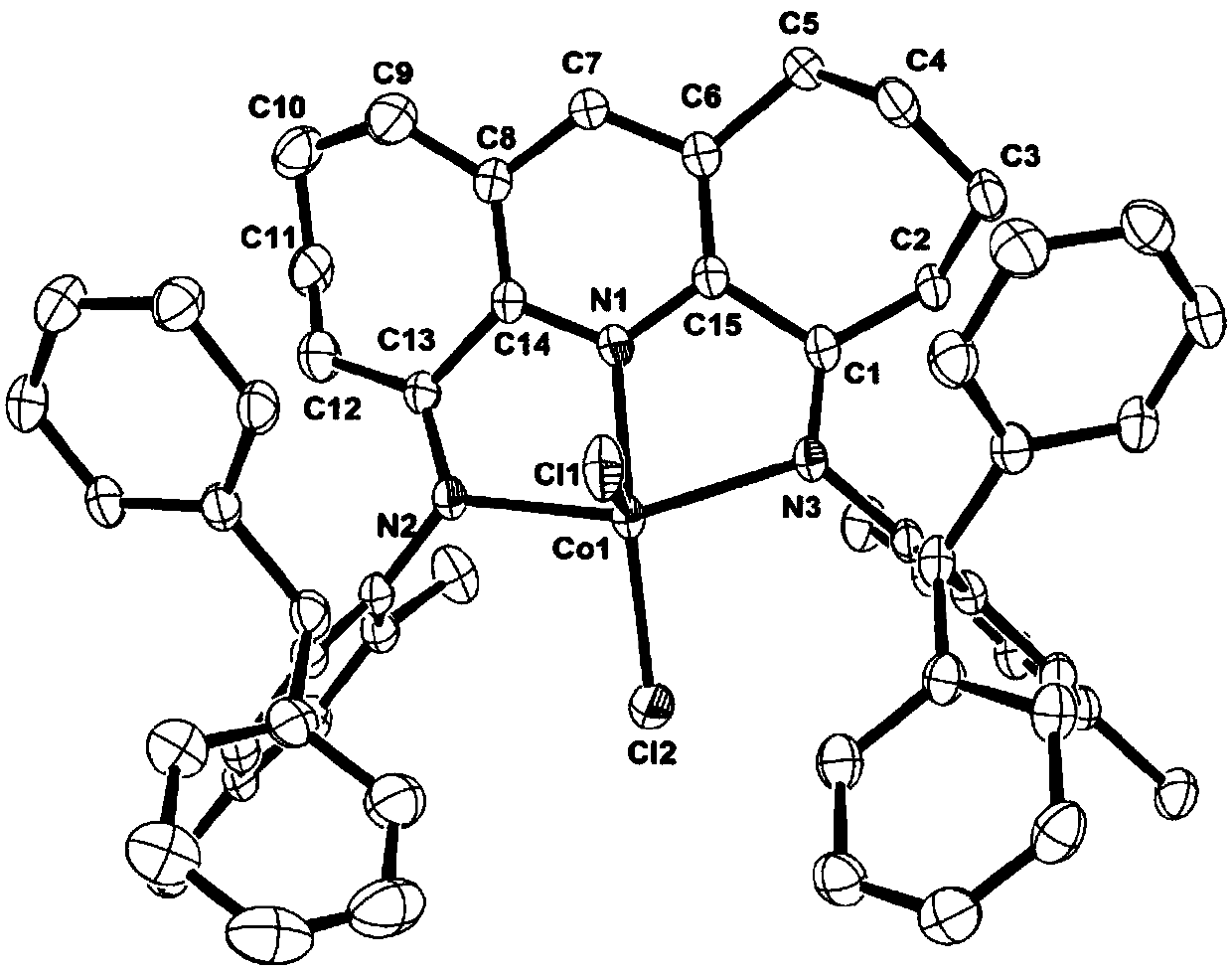
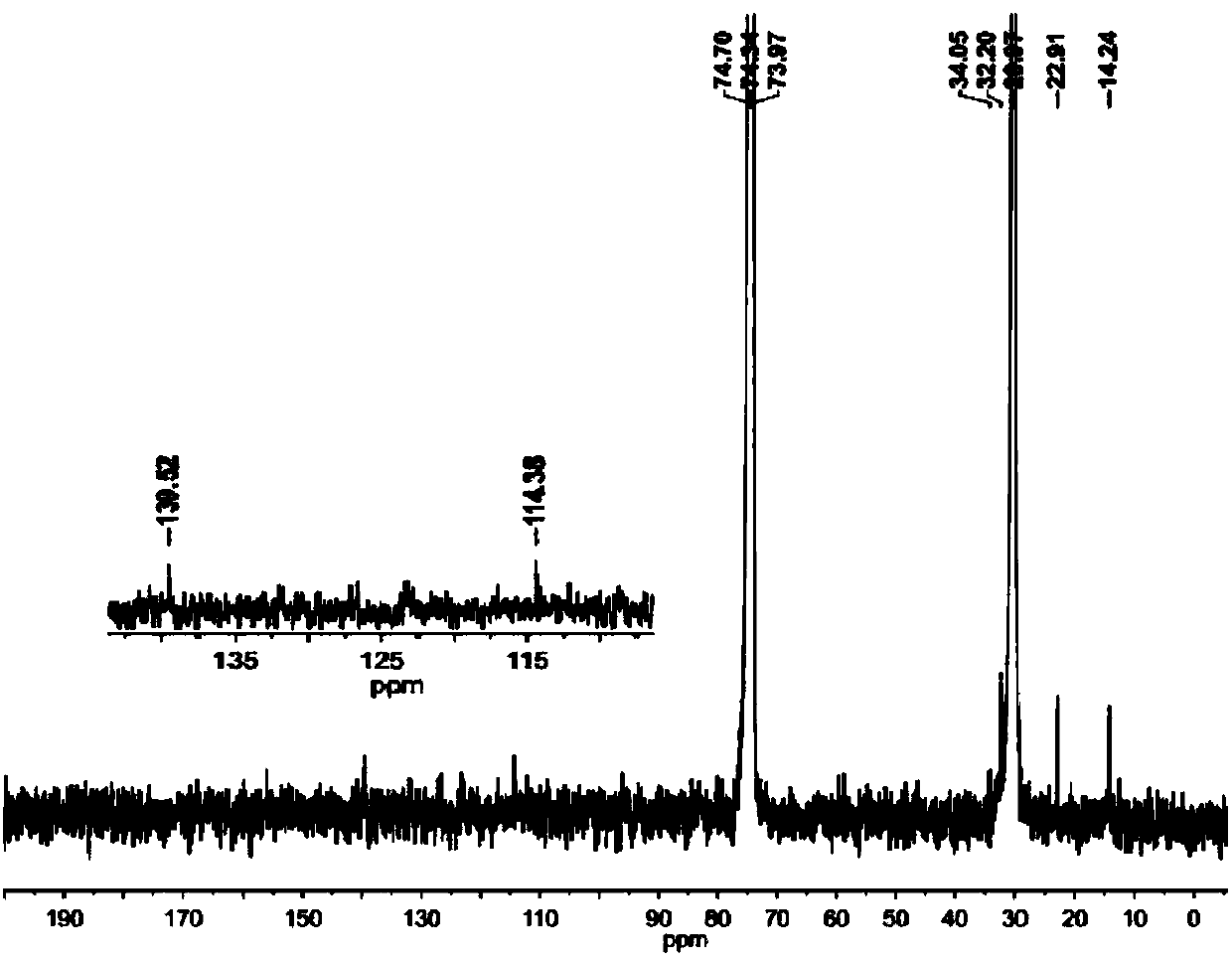
Symmetrical seven-membered imino pyridine complex containing large steric hindrance substituent groups for preparing polyethylene wax, and preparation method and application of symmetrical seven-membered imino pyridine complex
A complex and alkyl technology, applied in the field of symmetrical seven-membered ring pyridineimine complexes, can solve the problem that polyethylene cannot be used as polyethylene wax, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1. Preparation of α,α'-bis(2,4-dimethyl-6-benzhydrylbenzimine)-2,3:5,6-bis(pentamethylene)pyridine dichloride Ferrous[Fe1](R 1 is methyl, R 2 is methyl, R 3 for benzhydryl)
[0081]
[0082] 0.234g (1.0mmol) α,α'-dioxo-2,3:5,6-bis(pentamethylene)pyridine compound represented by formula (II-1), 1.15g (4.0mmol) 2,4 -Dimethyl-6-benzhydrylaniline and 0.199g (1.0mmol) FeCl 2 4H 2 O was dissolved in 10 mL of acetic acid, under nitrogen atmosphere, heated and stirred to reflux for 6 h, the reaction solution was concentrated, a large amount of ether was added, precipitated, and the precipitate was collected by filtration, then the precipitated substrate was dissolved in methanol, the solution was concentrated to the minimum volume, and again A large amount of diethyl ether was added and a precipitate was collected by filtration and washed with a large amount of diethyl ether. After drying, a light green powder (0.695 g, 76.5%) was obtained, which was Fe1 comple...
Embodiment 2
[0086] Example 2. Preparation of α,α'-bis(2-methyl-4,6-bis(benzhydryl)phenylimine)-2,3:5,6-bis(pentamethylene)pyridine bis Cobaltous chloride [Fe2] (R 1 is methyl, R 2 , R 3 for benzhydryl)
[0087]
[0088] Basically the same as the method in Example 1, the difference is that: under the condition of keeping the same molar number of reactants, the aniline participating in the reaction is 2-methyl-4,6-bis(benzhydryl)aniline, and light green is obtained after drying Powder (0.485g, 40%) is Fe2 complex.
[0089] The structural confirmation data are as follows:
[0090] FT-IR (KBr, cm -1 ): 2868(w), 1597(ν C=N )(s), 1555(w), 1494(s), 1447(s), 1338(m), 1256(s), 1207(m), 1151(m), 1077(m), 1030(m), 998(w), 969(m), 828(w), 778(w), 743(w), 698(s).
[0091] Elemental Analysis: C 81 h 71 Cl 2 FeN 3 (1213.23) Theoretical value: C, 80.19; H, 5.90; N, 3.46%. Experimental value: C, 80.00; H, 5.85; N, 3.35%.
Embodiment 3
[0092] Example 3. Preparation of α,α'-bis(2-ethyl-4,6-bis(benzhydryl)phenylimine)-2,3:5,6-bis(pentamethylene)pyridine bis Cobaltous chloride [Fe3] (R 1 is ethyl, R 2 , R 3 for benzhydryl)
[0093]
[0094] Basically the same as the method in Example 1, the difference is that: under the condition of keeping the same molar number of reactants, the aniline participating in the reaction is 2-ethyl-4,6-bis(benzhydryl)aniline, and light green is obtained after drying The powder (0.5g, 40.3%) is the Fe3 complex.
[0095] The structural confirmation data are as follows:
[0096] FT-IR (KBr, cm -1 ): 2871(w), 1630(m), 1596(ν C=N )(s), 1555(w), 1494(m), 1447(s), 1338(w), 1321(w), 1256(m), 1206(m), 1174(w), 1146(w), 1077(w), 1031(w), 1002(w), 969(w), 914(w), 850(w), 783(w), 743(w), 698(s)cm -1 .
[0097] Elemental Analysis: C 83 h 75 Cl 2 FeN 3 (1241.28) Theoretical: C, 80.31; H, 6.09; N, 3.39%. Experimental: C, 80.7; H, 5.08; N, 3.45%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Polymerization activity | aaaaa | aaaaa |
| Polymerization activity | aaaaa | aaaaa |
| Polymerization activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com