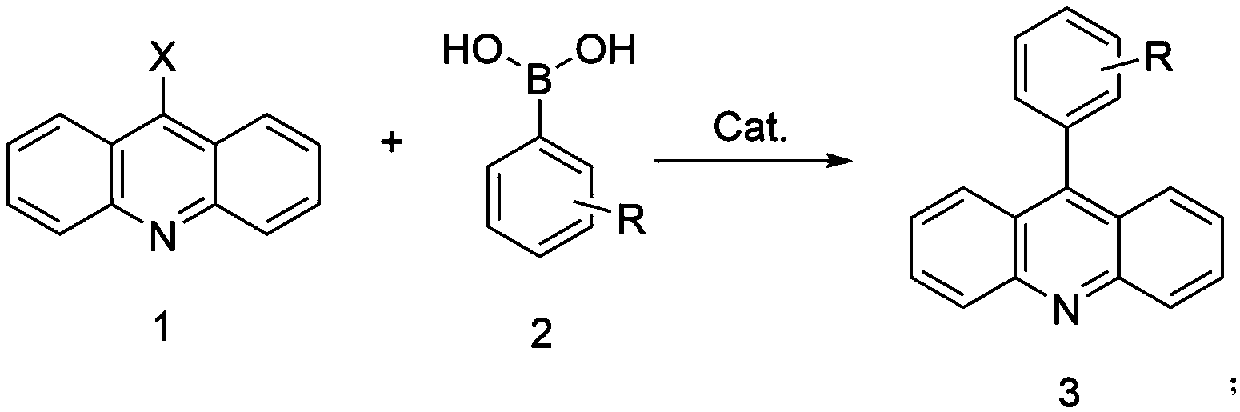
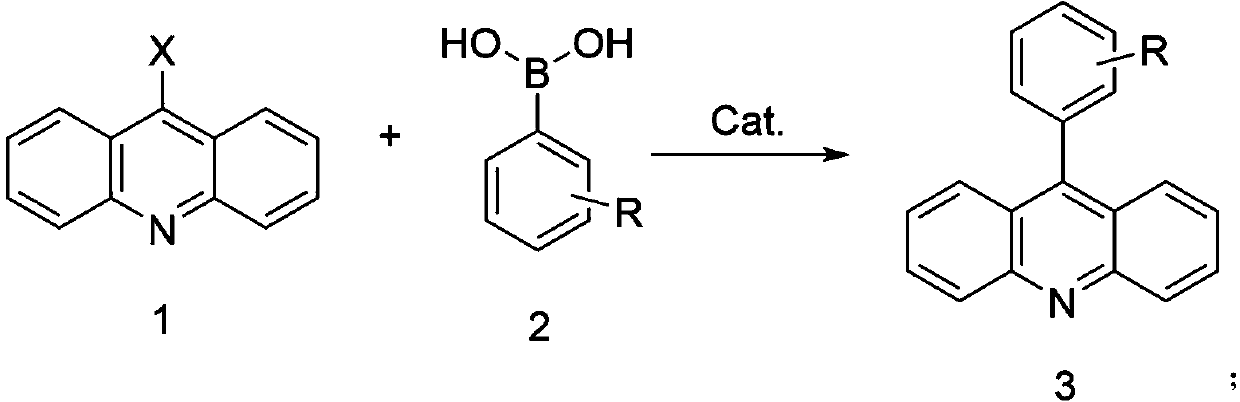
Method for preparing 9-phenylacridine compound
A technology for phenylacridine compounds, which is applied in the field of preparation of 9-phenylacridine compounds, can solve the problems of limiting the application of acridine photoinitiators, low reaction conversion rate, and low crude product content, and facilitates production The effect of selection, high content and short process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In a 250mL three-necked flask, 21.4g (0.1mol) of 9-chloroacridine, 14.6g (0.12mol) of phenylboronic acid, Pd(PPh 3 ) 4 Catalyst 0.11g, toluene 100g, sodium carbonate 2g, deionized water 18g, heated to 40°C, reacted for 3 hours, cooled to 25°C, removed the solvent by rotary evaporation, added 100g methanol to wash and disperse, and suction filtered to obtain the crude product, which was washed with 80g toluene After refining and drying, 24.7 g of the product was obtained, with a yield of 96.8% and an HPLC content of 99.65%. Elemental analysis: Cal: C89.38, H5.13, N5.49 Found: C89.35, H 5.11, N5.48; ESI: 255.31.
Embodiment 2
[0033] In a 250mL three-necked flask, add 21.4g (0.1mol) of 9-chloroacridine, 16.3g (0.12mol) of 3-methylphenylboronic acid, Pd(OAc) 2 Catalyst 0.11g, toluene 100g, sodium carbonate 2g, deionized water 18g, heated to 40°C, reacted for 3 hours, cooled to 25°C, removed the solvent by rotary evaporation, added 100g methanol to wash and disperse, and suction filtered to obtain the crude product, which was washed with 80g toluene Refined and dried to obtain 26.1 g of the product with a yield of 96.9% and an HPLC content of 99.69%. Elemental analysis: Cal: C89.19, H5.61, N5.20 Found: C89.17, H 5.63, N5.21; ESI: 269.33.
Embodiment 3
[0035] In a 250mL three-neck flask, add 21.4g (0.1mol) of 9-chloroacridine, 20.7g (0.14mol) of 3-vinylphenylboronic acid, and PdCl 2 Catalyst 0.12g, toluene 100g, potassium carbonate 2g, deionized water 18g, heated to 50°C, reacted for 4 hours, cooled to 25°C, removed the solvent by rotary evaporation, added 100g methanol to wash and disperse, obtained crude product by suction filtration, and used 80g toluene for the crude product Refined and dried to obtain 27.1 g of the product with a yield of 96.5% and an HPLC content of 99.58%. Elemental analysis: Cal: C89.65, H 5.37, N4.98 Found: C89.64, H 5.35, N4.99; ESI: 281.34.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com