Self-supporting nanoporous nitrogen reducing catalyst and preparation method thereof
A nanoporous, self-supporting technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve problems such as limited stability, and achieve easy operation, simple process flow, and improved catalytic activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
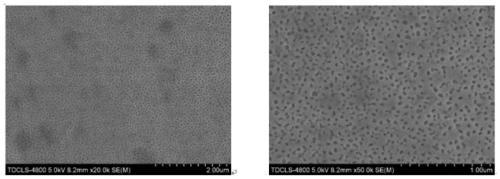
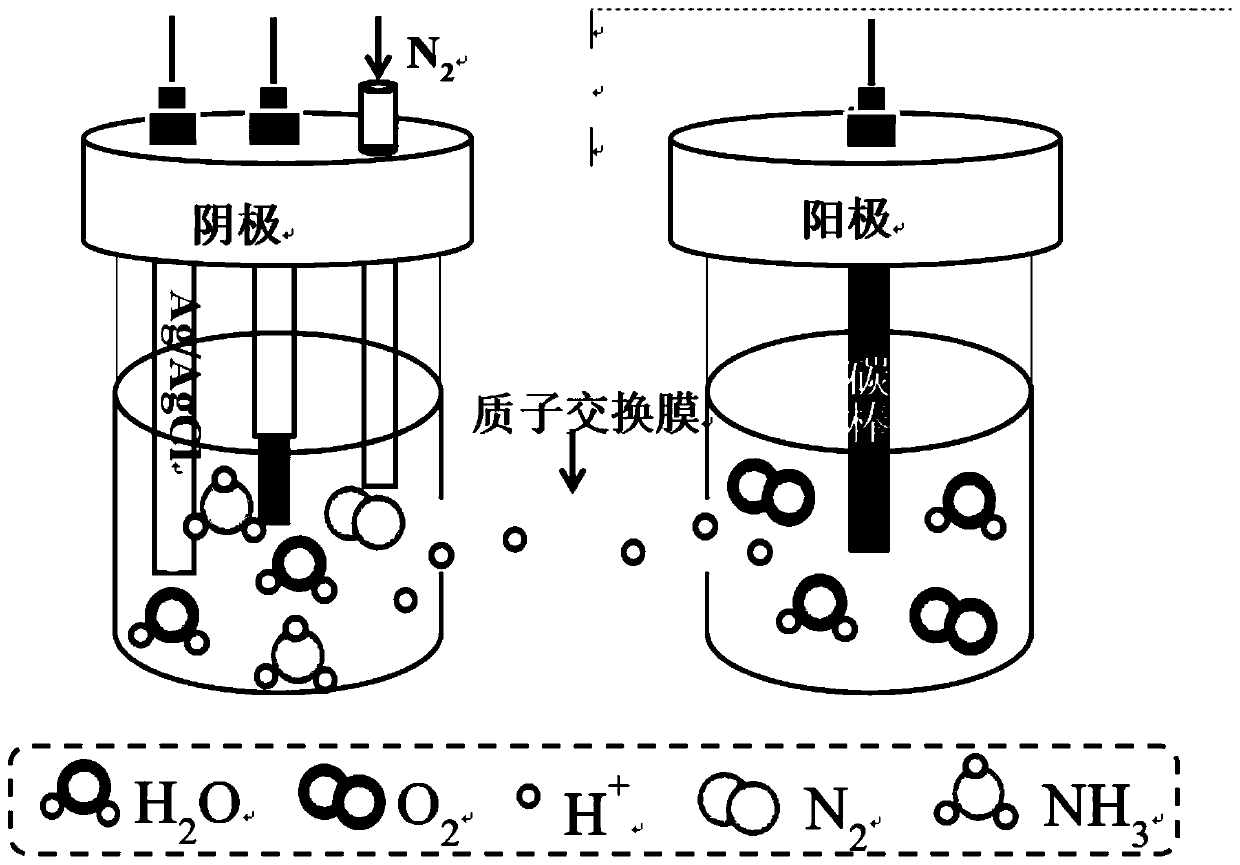
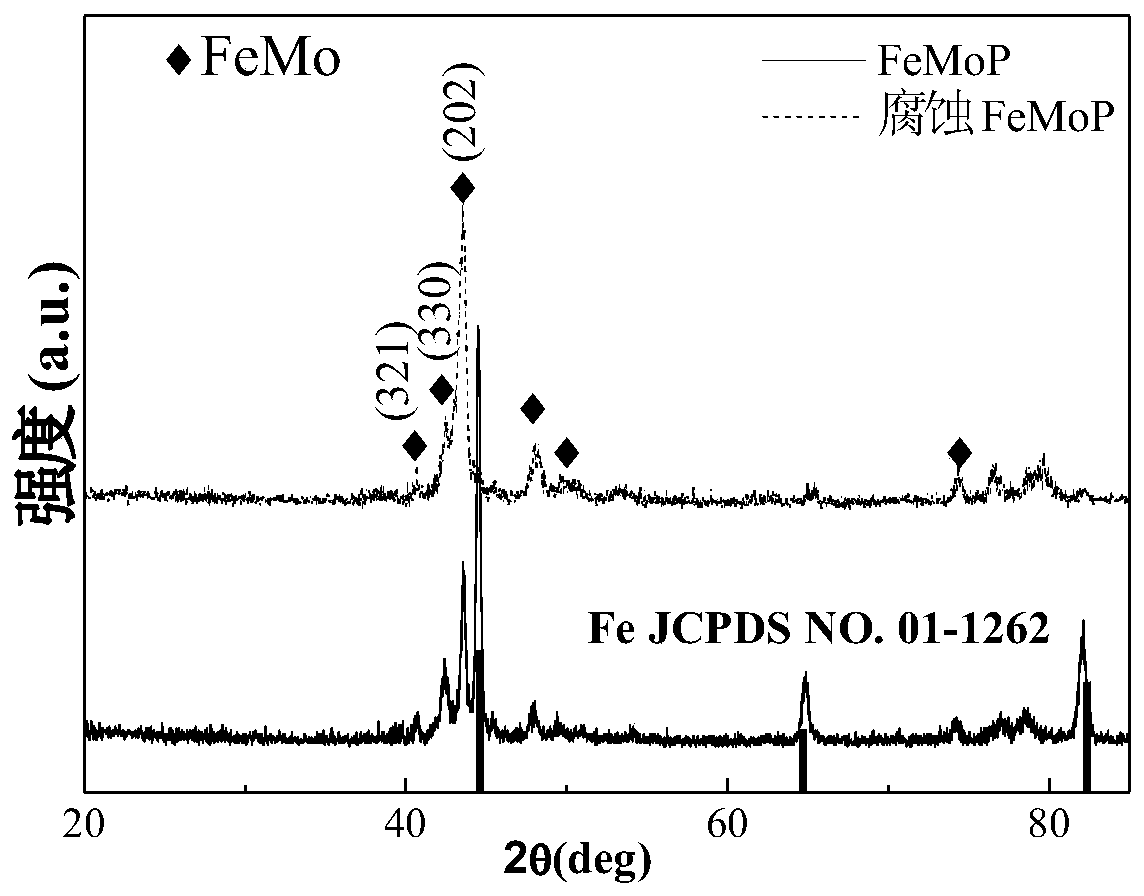
[0026] Step 1, get pure iron (Fe) 4.8674g, pure iron triphosphorus (Fe 3 P) 3.4603g, pure molybdenum (Mo) 1.6723g, a total of 10g, 10g samples were mixed evenly, placed in an argon melting furnace, and vacuumed by a mechanical pump to 1×10 -1 After Pa, turn off the mechanical pump and turn on the molecular pump to draw a high vacuum to 2.3×10 -3 Finally, the molecular pump is turned off, and the high-purity argon protective gas is introduced. The melting time of the raw material is 160s, and the front and back are smelted 4-5 times, so that the internal components of the alloy are fully mixed and evenly obtained to obtain Fe 80 Mo 10 P 10 alloy ingot.
[0027] Step 2, after cooling down sufficiently, take out the alloy ingot and cut it into pieces, take 3g and ultrasonically clean and dry it with anhydrous ethanol, place it in a clean quartz tube, install it in a belt spinner, and evacuate it to 7.8× 10 -3 After Pa, turn off the vacuum, adjust the speed of the copper roll...
Embodiment 2
[0036] Step 1, according to the mass percent of pure Fe 40%, the mass percent of pure Mo 10%, Fe 3 The mass percentage of P is 50% for batching, placed in the argon smelting furnace, and the mechanical pump is vacuumed to 1×10 -1 After Pa, turn off the mechanical pump and turn on the molecular pump to draw a high vacuum to 2.0×10 -3 Finally, the molecular pump is turned off, and the high-purity argon protective gas is introduced. The melting time of the raw material is 120s, and the front and back are smelted 4-5 times, so that the internal components of the alloy are fully mixed and uniform, and the Fe-Mo-P alloy ingot is obtained.
[0037] Step 2, after fully cooling, take out the alloy ingot and cut it into pieces, take 3g and ultrasonically clean and dry it with absolute ethanol, place it in a clean quartz tube, install it in a belt spinner, and evacuate it to 7× 10 -5 After Pa, turn off the vacuum, adjust the speed of the copper roller to 1000 rpm, control the pressure ...
Embodiment 3
[0040] Step 1, according to the mass percent of pure Fe 60%, the mass percent of pure Mo 10%, Fe 3 The mass percentage of P is 30% for batching, placed in the argon smelting furnace, and the mechanical pump is vacuumed to 1×10 -1 After Pa, turn off the mechanical pump and turn on the molecular pump to pump a high vacuum to 3.0×10 -3 Finally, the molecular pump is turned off, high-purity argon protective gas is introduced, the melting time of the raw material is 240s, and the front and back are smelted 4-5 times, so that the internal components of the alloy are fully mixed and uniform, and the Fe-Mo-P alloy ingot is obtained.
[0041] Step 2, after cooling down sufficiently, take out the alloy ingot and cut it into pieces, take 3g of it and ultrasonically clean it with absolute ethanol and blow it dry, put it in a clean quartz tube, install it in a belt spinner, and evacuate it to 8× 10 -5 After Pa, turn off the vacuum, adjust the speed of the copper roller to 3000 rpm, contr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com