Amorphous iron-nickel phosphonate with efficient photocatalysis oxygen production property and preparation method and application of amorphous iron-nickel phosphonate
A photocatalytic and amorphous technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., can solve the problem of low photocatalytic oxygen production efficiency and achieve technological Simple operation, high-efficiency photocatalytic oxygen production performance, and high repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Dissolve 1 mmol of iron-nickel nitrate in 5 ml of N,N-dimethylformamide (solution A), and dissolve 1 mmol of 2-carboxyethylphosphonic acid in 10 ml of N,N-dimethylformamide (solution B). Add solution A to solution B dropwise under magnetic stirring. After reacting for 12 hours under solvothermal conditions at 140° C., the sample was washed by centrifugation and collected to obtain amorphous iron-nickel phosphonate.
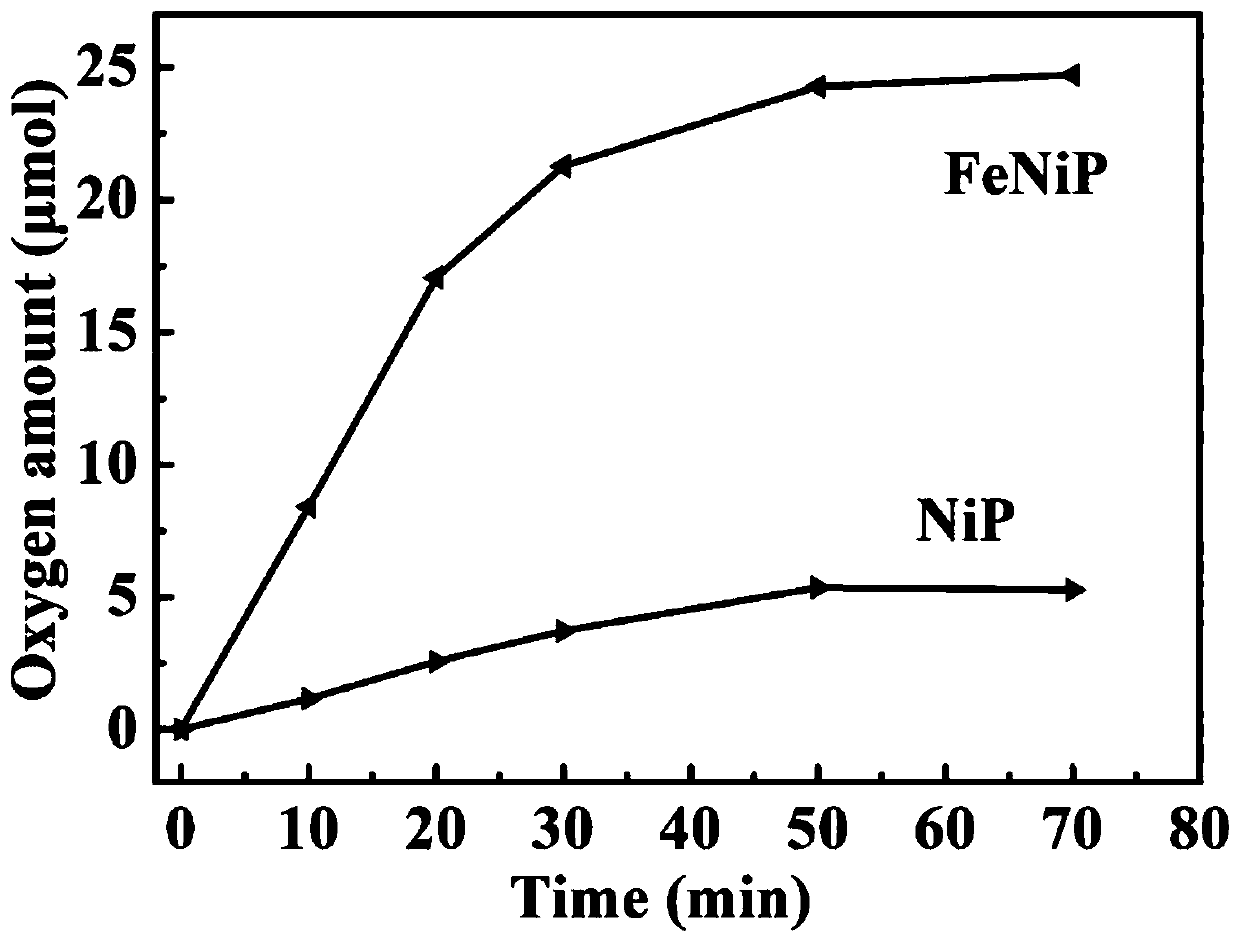
[0023] The photocatalytic performance of the amorphous iron-nickel phosphonate prepared in Example 1 was measured in a closed gas circulation system, and the reaction system was kept at about 20° C. by circulating cooling water. With photocatalyst (the amorphous iron nickel phosphonate that embodiment 1 makes, nickel phosphate), [Ru(bpy) 3 ] Cl 2 ·6H 2 O and Na 2 S 2 o 8 Mix in borate buffer. A 300w xenon lamp was selected as the light source, and the reaction system was backfilled with argon several times to remove air before illumination. Determin...
Embodiment 2
[0026] Dissolve 2mmol of iron-nickel nitrate in 15ml N,N-dimethylformamide (solution A), dissolve 2mmol of 2-carboxyethylphosphonic acid in 15ml N,N-dimethylformamide (solution B), in Add solution A to solution B dropwise under magnetic stirring. After reacting for 24 hours under solvothermal conditions at 160° C., the sample was washed by centrifugation and collected to obtain amorphous iron-nickel phosphonate.
Embodiment 3
[0028] Dissolve 3mmol of iron-nickel nitrate in 25ml of N,N-dimethylformamide (solution A), and dissolve 4mmol of 2-carboxyethylphosphonic acid in 30ml of N,N-dimethylformamide (solution B). Add solution A to solution B dropwise under magnetic stirring. After reacting for 36 hours under solvothermal conditions at 200° C., the samples were washed by centrifugation and collected to obtain amorphous iron-nickel phosphonate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com