Air purification material and preparation method thereof
A technology for air purification materials and substrates, which is applied in the field of air purification and can solve problems such as limiting the scope of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
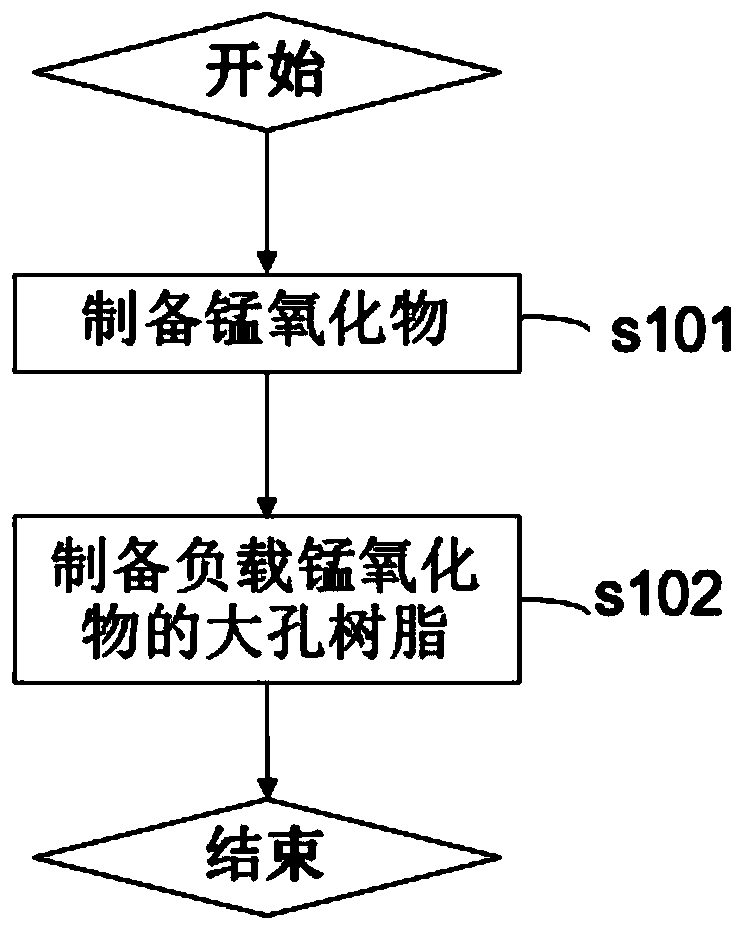
[0031] First, refer to figure 1 , which shows a flow chart of preparing an air-purifying material according to the present invention. Such as figure 1 As shown, in step s101, manganese oxide capable of decomposing formaldehyde is prepared. Manganese oxide can effectively decompose formaldehyde at normal temperature, and is a good material for decomposing formaldehyde. Subsequently, in step s102, the prepared manganese oxide is loaded onto a macroporous resin as a substrate to prepare a macroporous resin loaded with manganese oxide, and the air purification material according to the present invention can be obtained.
[0032] Hereinafter, the method for preparing an air-purifying material according to the present invention will be specifically described.
[0033] Manganese oxide preparation
[0034] According to one embodiment, the manganese oxide according to the present invention may be a birnessite-type manganese oxide, and its specific preparation process is as follows...
no. 2 example
[0066] Hereinafter, differences from the method for preparing an air-purifying material of the first embodiment will be mainly described, and redundant descriptions of the same parts as those of the method for preparing an air-purifying material of the first embodiment will be omitted.
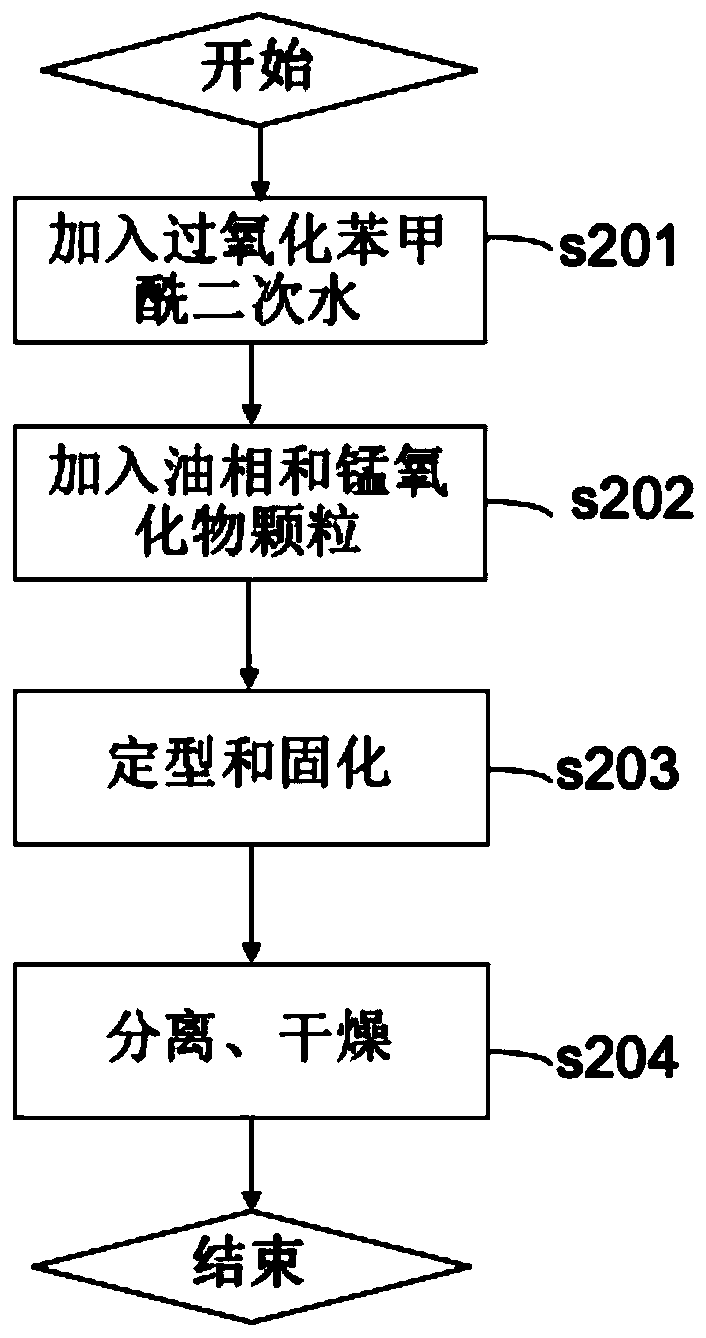
[0067] Such as Figure 4 As shown, in the method for preparing an air purification material according to the second embodiment of the present invention, a step s103 is further included, and the step s103 softens the obtained macroporous resin loaded with manganese oxide.
[0068] As mentioned above, the macroporous resin loaded with manganese oxide prepared according to the method described in the first embodiment is in the form of particles. The granular state limits its range of use. Specifically, in many cases, the resulting formaldehyde-decomposing material needs to be compliant so that it can be formed into various shapes. For example, in the case where formaldehyde needs to be removed ...
no. 3 example
[0084] Hereinafter, the differences from the methods of preparing the air-purifying material of the first and second embodiments will be mainly described, and redundant descriptions of the same parts as those of the methods of preparing the air-purifying material of the first and second embodiments will be omitted.
[0085] Since the boiling point of formaldehyde is -19.5 degrees Celsius, formaldehyde exists in the air in gaseous form at room temperature. On the other hand, formaldehyde is very soluble in water, so most of the formaldehyde in the air exists in the form of combining with water molecules. In view of this, formaldehyde dissolved in water in the air can be decomposed by increasing the affinity of the air purification material with water. In fact, most formaldehyde in the air exists in the form of being dissolved in water, and decomposing formaldehyde dissolved in water can greatly improve the decomposition effect of formaldehyde.
[0086]Specifically, in this exe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com