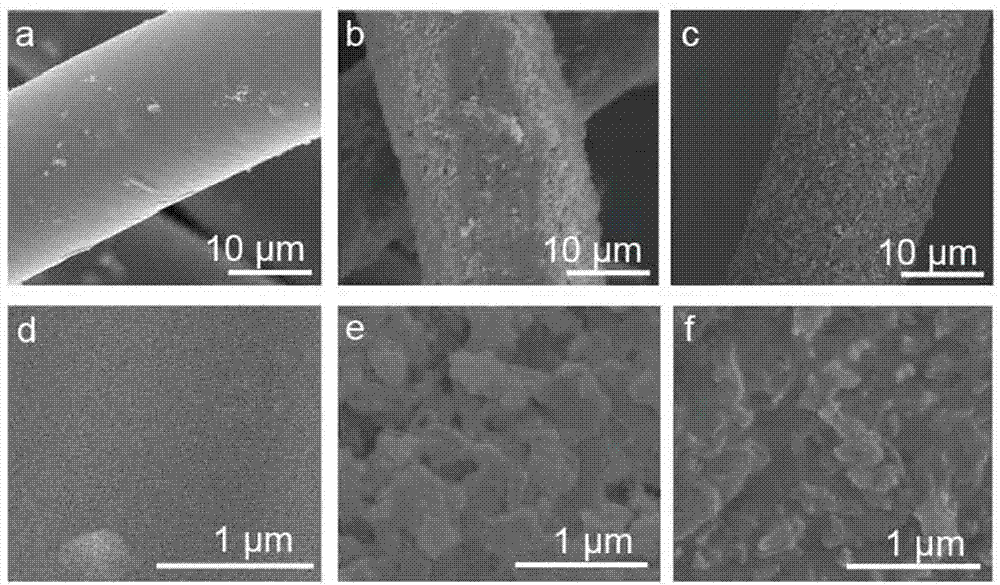
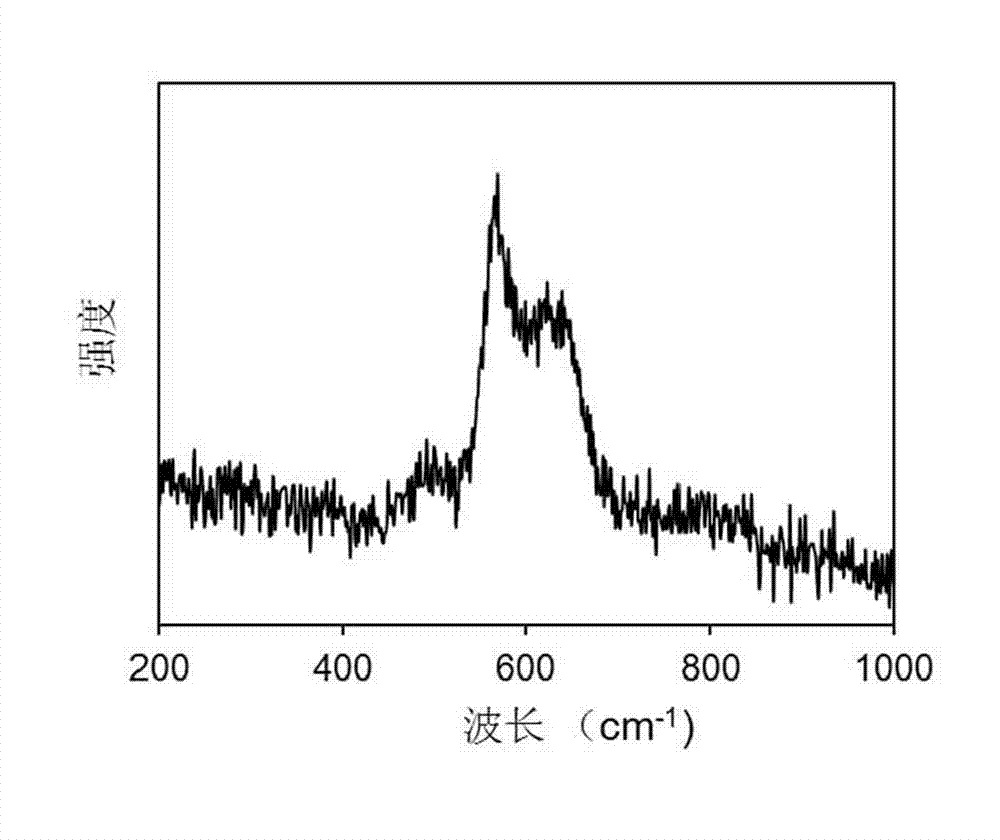
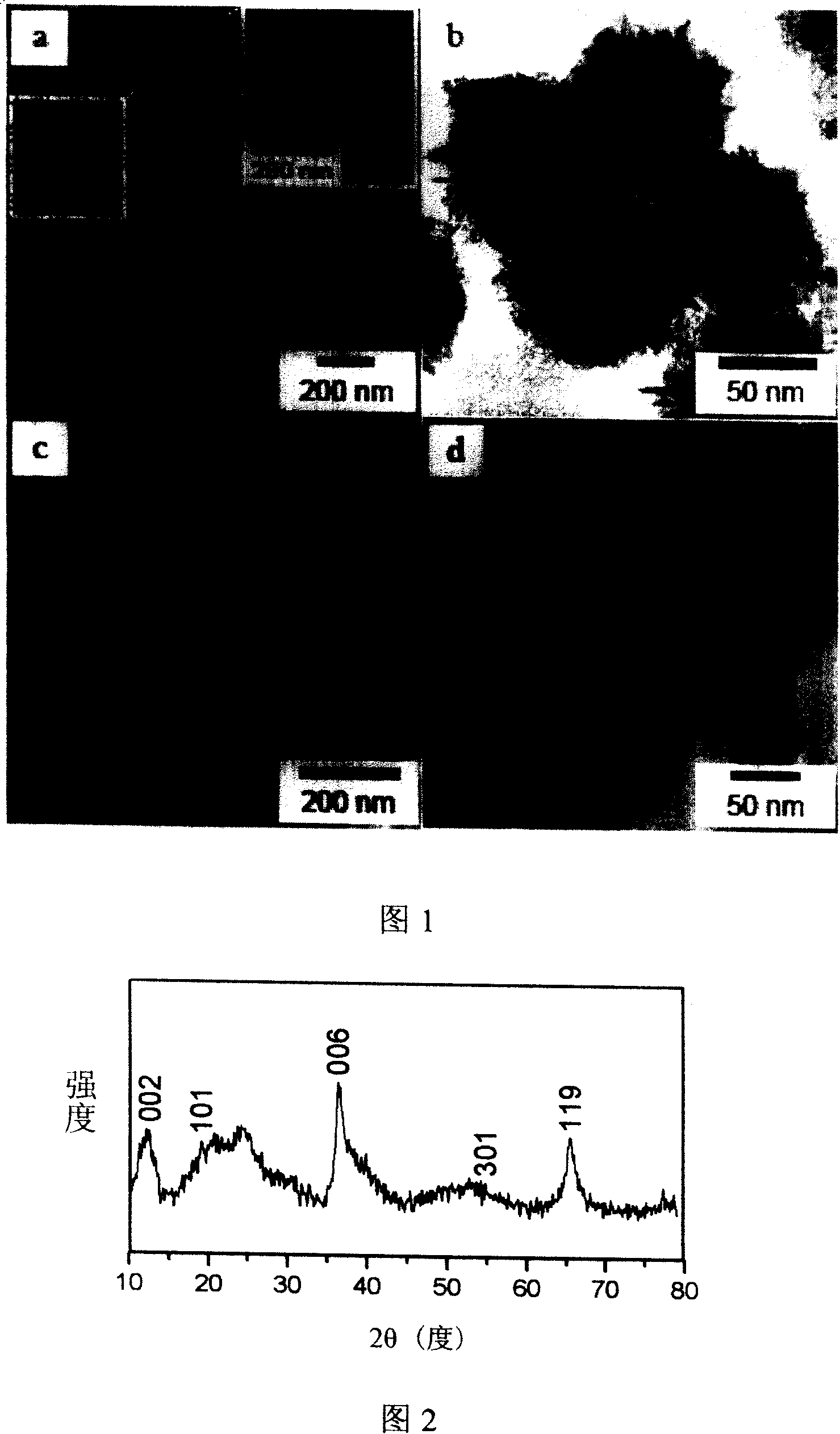
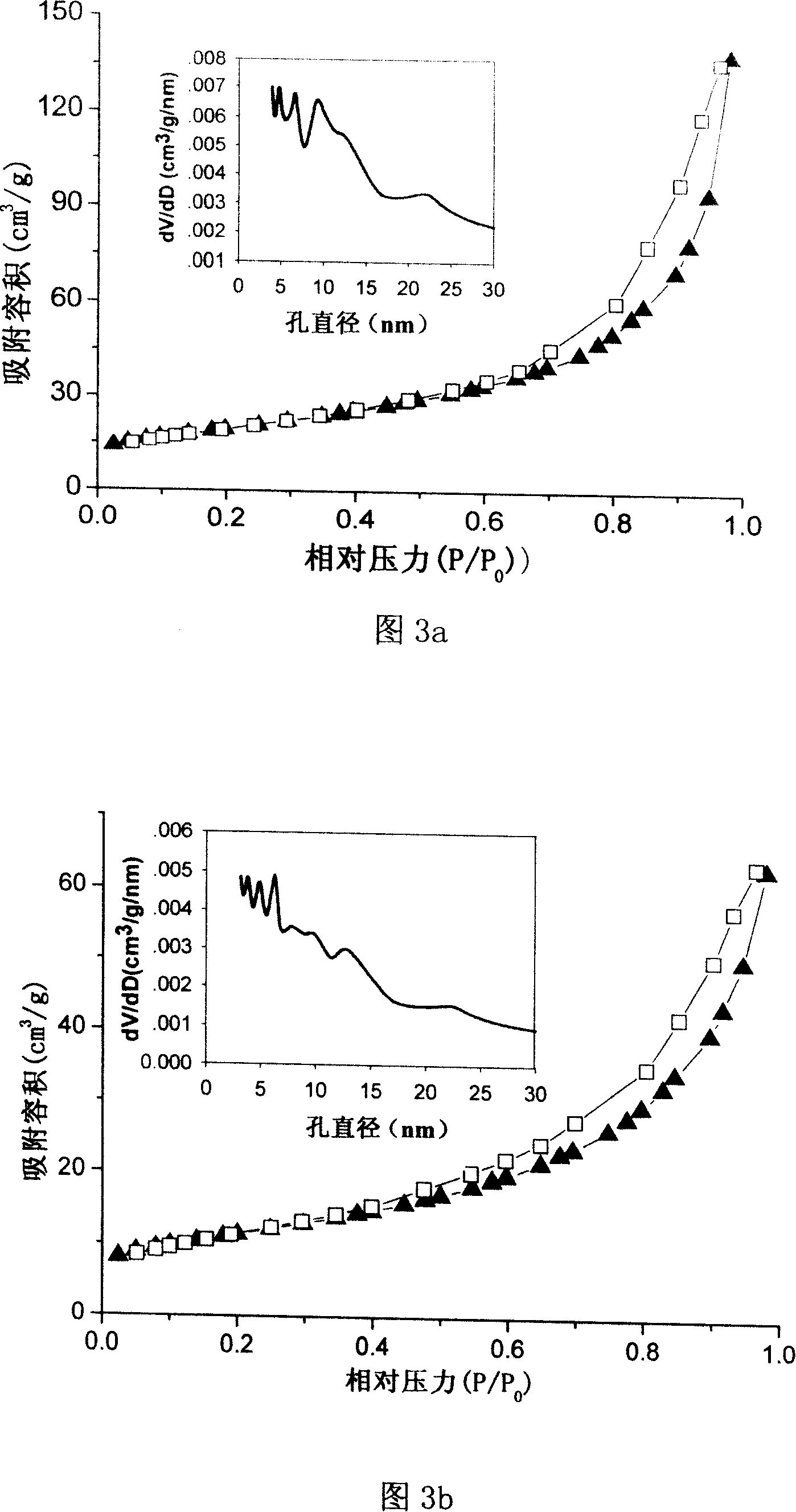
Patents
Literature
82 results about "Birnessite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
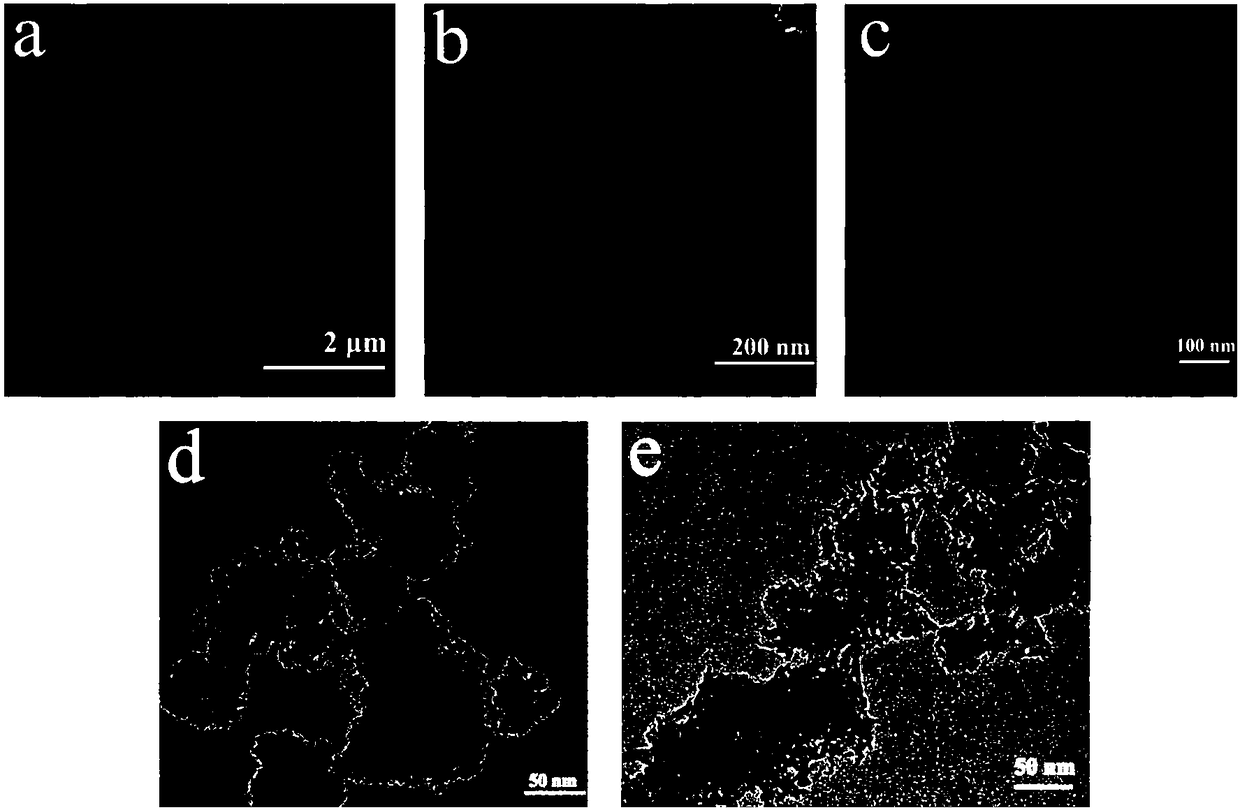
Birnessite (Na0.3Ca0.1K0.1)(Mn⁴⁺,Mn³⁺)₂O₄ · 1.5 H₂O is an oxide mineral of manganese along with calcium, potassium and sodium. It has a dark brown to black color with a submetallic luster. It is also very soft, with a Mohs hardness of 1.5. Birnessite is formed by precipitation in lakes, oceans and groundwater and is a major component of desert varnish and deep sea manganese nodules.
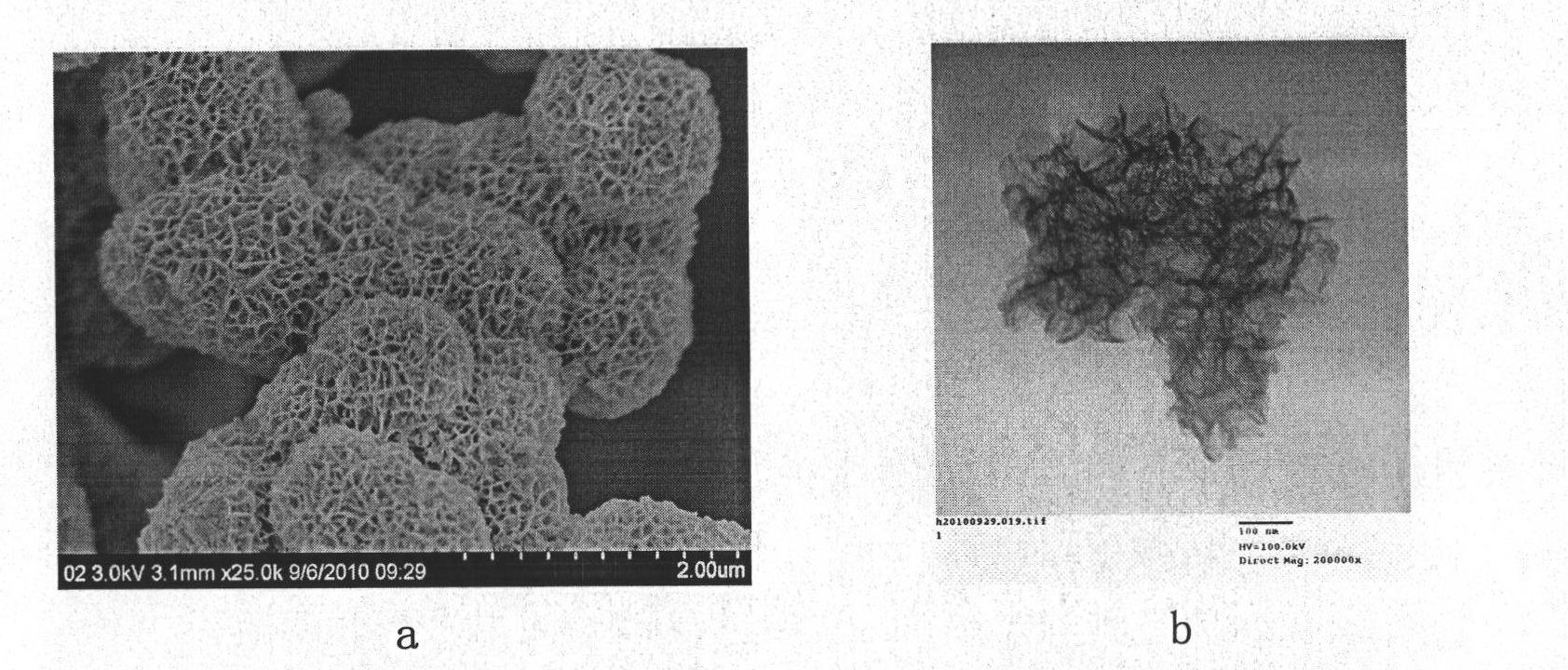
Preparation method of brain-coral-shaped birnessite type manganese dioxide
InactiveCN102120619AImprove structural stabilityShape unchangedNanotechnologyManganese oxides/hydroxidesStructural stabilityPotassium permanganate
Owner:HEBEI NORMAL UNIV
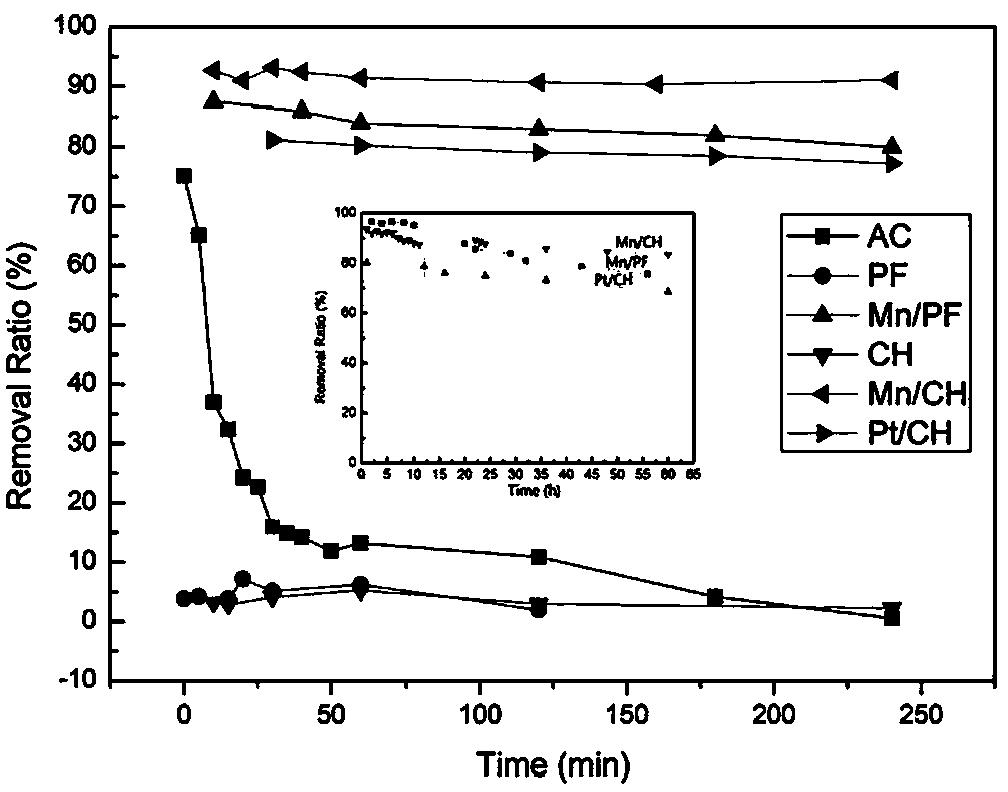
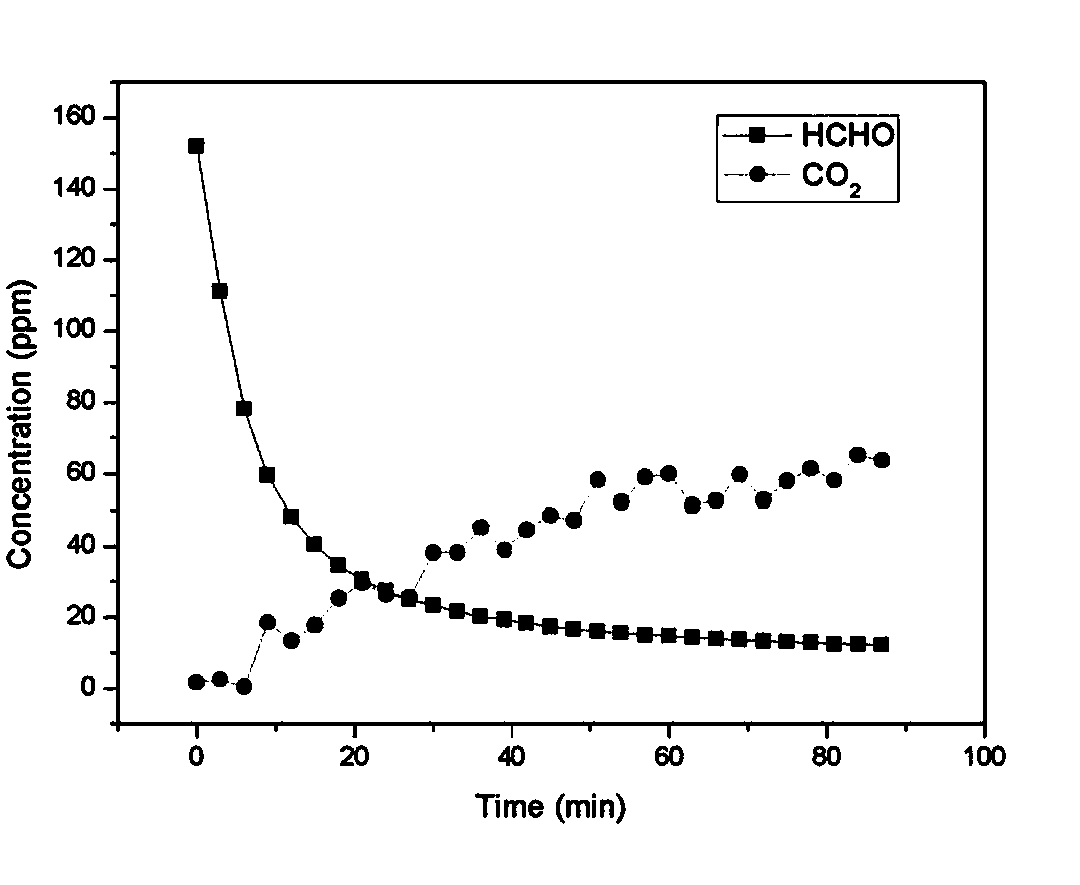
Air purification material and preparation method and application thereof
ActiveCN104190251AEfficient decompositionQuick removalDispersed particle separationFiberParticulates
The invention relates to an air purification material and a preparation method and application thereof, belonging to the technical field of chemical catalytic decomposition, in particular belonging to the technical field of decomposition of formaldehyde pollutants in environment air. The air purification material comprises a base material and manganese oxide, wherein the manganese oxide is supported on the base material, the base material is honeycomb ceramics or a fiber material with a particle filtering function, and the manganese oxide is birnessite manganese oxide prepared from permanganate and oxalate. The air purification material can effectively decompose formaldehyde pollutants in indoor air and can rapidly and constantly remove the formaldehyde pollutants in indoor air at room temperature. The air purification material can be regenerated through heating, so that the service life of the material is prolonged and the practical application is facilitated.
Owner:TSINGHUA UNIV
Air cleaning material, and preparation method and application thereof
ActiveCN103480267ASimple preparation processLow costDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsFiberParticulates
The invention relates to an air cleaning material, and a preparation method and an application thereof, and belongs to the technical field of chemical catalytic decomposition. The air cleaning material comprise a base material and a manganese oxide loaded on the base material; the manganese oxide is a birnessite type manganese oxide prepared by performing in-situ reduction on a permanganate and a reducing agent; and the base material is filter cotton, non-woven fabrics, cotton cloth, gauze, or fiber with particle filtering function. The preparation method comprises the following steps: step 1, dissolving a quaternary ammonium salt and the permanganate in water, adding the base material; step 2, adding the reducing agent into the solution obtained in the step 1, mixing uniformly; step 3, heating the solution obtained in the step 2 at a constant temperature; and step 4, taking out the base material and drying to obtain the finished product. According to the technical scheme, the manganese oxide is simple loaded on the fiber material base material such as filter cotton and the like, so that the obtained low-wind-resistance cleaning material is capable of continuously rapid degrading formaldehyde and ozone in the air at room temperature; and the preparation method of the air cleaning material is simple and low in cost, and no other pollutions are introduced.
Owner:TSINGHUA UNIV
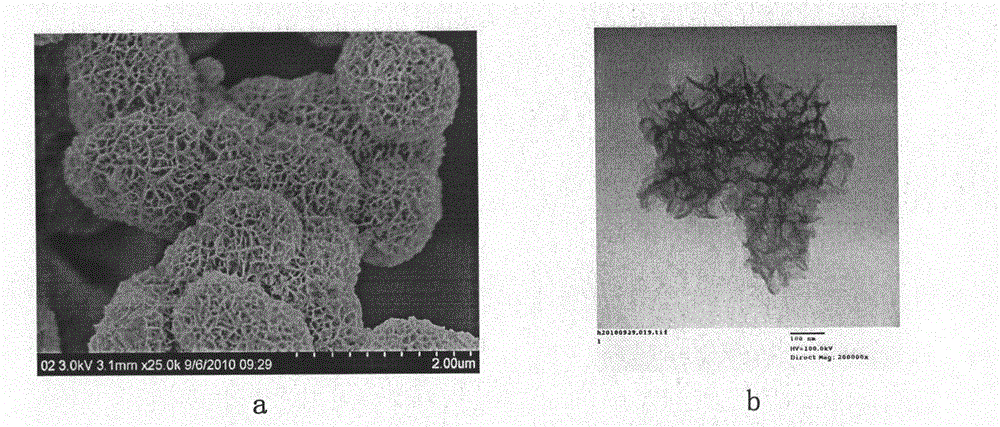
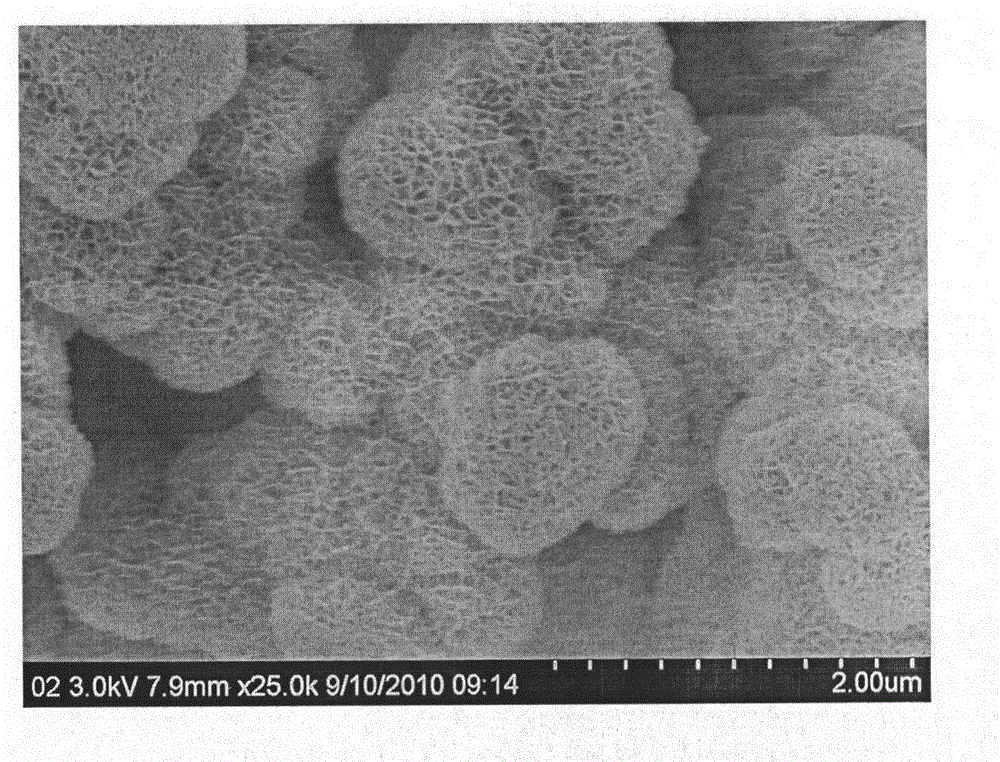
Layered mesoporous birnessite manganese dioxide cellular nano ball, preparing method and use of the same
InactiveCN101152962AEvenly distributedShape unchangedManganese oxides/hydroxidesSorbentStructural stability
The present invention belongs to the technology field of nanometer material preparing, in particular to a preparing method of layered meso porous water natrium manganese mine type MnO2 alveolate nanometer sphere and hollow nanometer sphere. The present invention takes cheap potassium permanganate and oleic acid as reactant. Acid or alkali processing is not needed. A redox takes place in a neutral aqueous solution. Through controlling a molar ratio of potassium permanganate and oleic acid, monodisperse layered meso porous water natrium manganese mine type MnO2 alveolate nanometer sphere and hollow nanometer sphere can be respectively obtained. The present invention does not need surfactant, is simple in process, low in cost and mild in reaction condition. The layered meso porous water natrium manganese mine type MnO2 alveolate nanometer sphere and hollow nanometer sphere of the present invention are rich in meso porous, big in specific surface area and hole capacity and good in structure stability, which can be used as vector of activator or sorbent. The present invention can also be used as separation material, magnetic material, battery electrode material, oxidative degradation material, desulfurization material or air purification material.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Use of layered mesoporous birnessite type MnO2 cellular nano-sphere
InactiveCN101209414AHigh catalytic activityRich in mesoporesDeodrantsMetal/metal-oxides/metal-hydroxide catalystsSorbentCatalytic oxidation
The invention belongs to the technical field of nanophase material preparation, in particular to faviform nano-spheres of lamellar mesoporous birnessite-manganese-dioxides MnO2 type which are used as catalysts for catalyzing and oxidizing formaldehyde completely under high-efficiency low temperature. The invention takes inexpensive potassium permanganate and oleic acid as reagents with no need of acid or alkali processing, leads redox reaction of the reagents to take place in a neutral aqueous solution, and controls the molar ratio between the potassium permanganate and the oleic acid, thus respectively obtaining monodisperse-faviform nano-spheres and hollow nano-spheres of lamellar mesoporous birnessite-manganese-dioxides. The monodisperse-faviform nano-spheres and the hollow nano-spheres of lamellar mesoporous birnessite-manganese-dioxides of the invention have rich mesopores, bigger specific surface and mesopore capacity, and good structural stability, and can be taken as carriers of catalysts or sorbents, separation materials, magnetic materials, electrode materials of batteries, oxidation and degradation materials, desulfidation or air purification materials, especially as catalysts for catalyzing and oxidizing formaldehyde completely at low temperature.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Cu-Ce doped type manganese oxide catalyst, preparation method and application thereof
InactiveCN102513122AImprove catalytic performanceGood catalyticDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsBirnessitePtru catalyst
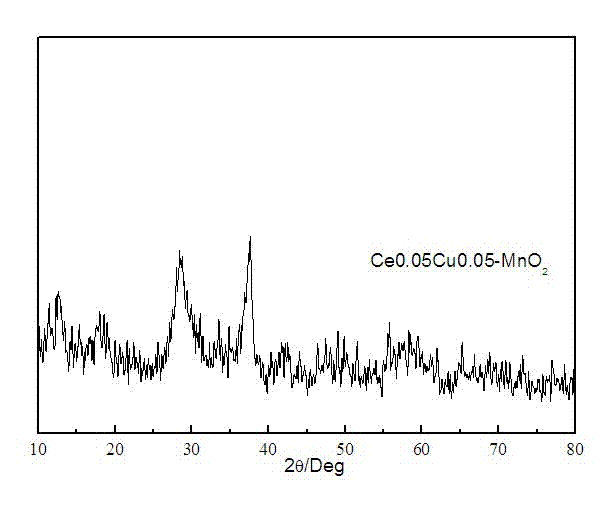
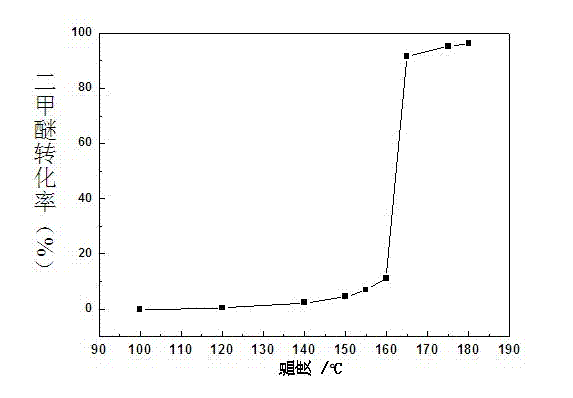
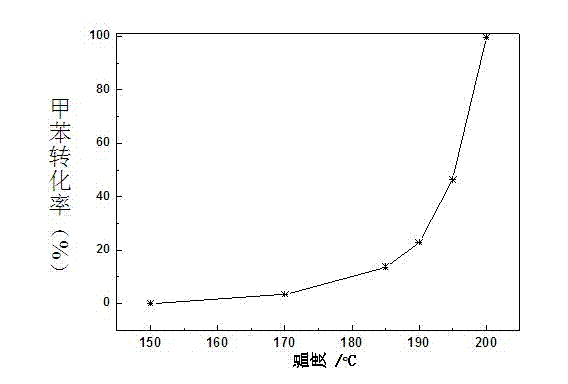
The invention discloses a Cu-Ce doped type manganese oxide catalyst, a preparation method and the application of the Cu-Ce doped type manganese oxide catalyst. The precursor Birnessite-type manganese oxide of the catalyst is firstly prepared by a sol-gel method, the components Ce and Cu are introduced at the same time by an immersion method, and finally, the Cu-Ce doped type manganese oxide catalyst can be obtained by roasting. The Cu-Ce doped type manganese oxide catalyst is low in price, has better adaptability, can be applied to the catalytic combustion of dimethyl ether and volatile organic compounds (VOCs), has excellent low-temperature activity, and only has the products of carbon dioxide and water without other by-products.
Owner:GUANGDONG UNIV OF TECH
Preparation method of sodium manganese oxide
ActiveCN103896339AAdvantages of preparation methodHigh purityCell electrodesSecondary cellsChemical reactionMolecular level
The invention relates to a preparation method of a sodium manganese oxide, and particularly relates to a preparation method of a layered birnessite type sodium manganese oxide. The preparation method is as follows: reacting a two valent manganese salt with sodium hydroxide in an aqueous phase to obtain a Mn (OH)2 precipitate and / or MnOOH precipitate-containing suspension, then performing solvent evaporation to obtain a MnOOH precipitate and / or Mn (OH) 2 precipitate-containing mixture; grinding the mixture, and sintering at 600-1200 DEG C to obtain a sintering product; then performing washing and solid-liquid separation for multiple times, and finally drying to obtain a final product. According to the preparation method, molecular level mixing of manganese hydroxide and a sodium salt can be realized by a solution chemical reaction method, promotion of next-step solid phase reaction is facilitated, improvement of the purity of the product is facilitated, and the prepared sodium manganese compound can be used in a sodium ion battery using an aqueous solution as an electrolyte, and helps to improve the charge discharge performance of the battery.
Owner:紫石能源有限公司
Method of preparing manganese oxide hollow nano-sphere with large-specific surface area
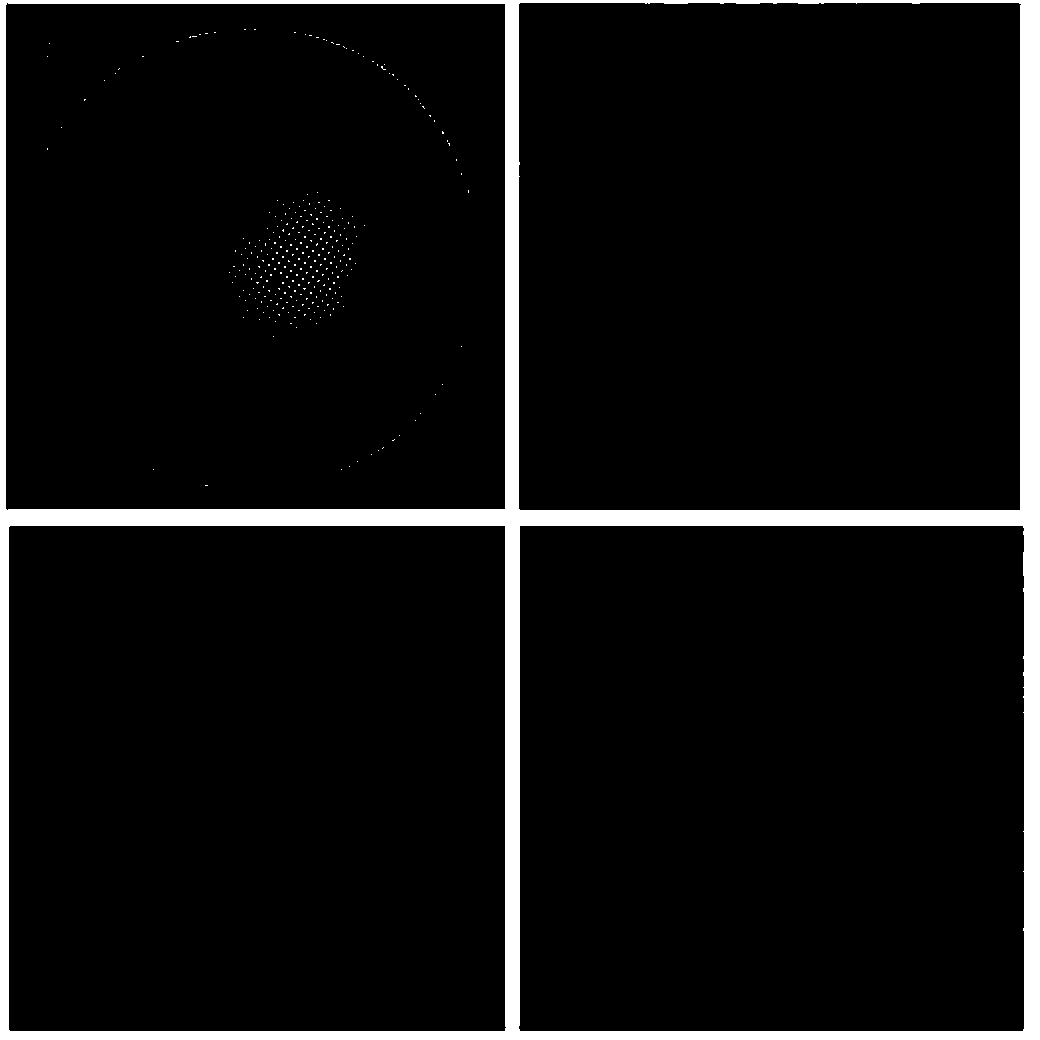
InactiveCN101343081AThe process steps are simpleReduce manufacturing costManganese oxides/hydroxidesFourier transform on finite groupsX-ray
Disclosed is a preparation method of a hollow manganese oxide nanosphere of large specific surface area, comprising steps of preparing a silicon dioxide nanosphere, preparing a silicon dioxide composite nanosphere covered by manganese dioxide and preparing hollow manganese oxide nanosphere. Because the preparation method adopts the process steps, which are simple, the production cost is low; the prepared hollow manganese oxide nanosphere is tested by an X-ray diffraction instrument, a scanning electron microscope, a transmission electron microscope, a Fourier transform infrared spectrometer and a physisorption instrument; the hollow manganese oxide nanosphere belongs to natrium-manganese crystal phase and is characterized by spherical morphology, having particle sizes ranging from 300 to 450nm and large specific surface area, and can be used as electrode materials to produce electrochemical capacitors.
Owner:SHAANXI NORMAL UNIV
Air purifying material and preparation method and application thereof
InactiveCN105854592AHigh upload rateNot easy to fall offGas treatmentDispersed particle separationBirnessiteRoom temperature
The invention relates to an air-purifying material, a preparation method and application thereof, and belongs to the technical field of catalyst materials. The material includes a base material and a manganese oxide, and the manganese oxide is supported on the base material. It is characterized in that the base material is aluminum or an aluminum alloy, and the manganese oxide is a birnessite-type manganese oxide. The invention has the advantages of simple coating process and easy large-scale production. The manganese oxide catalyst has a high loading rate on the surface of the aluminum substrate, is firm and not easy to fall off. The purification material of the invention can quickly decompose formaldehyde or ozone in the air at room temperature, has low purification cost, and can quickly regenerate the catalyst by heating.
Owner:TSINGHUA UNIV
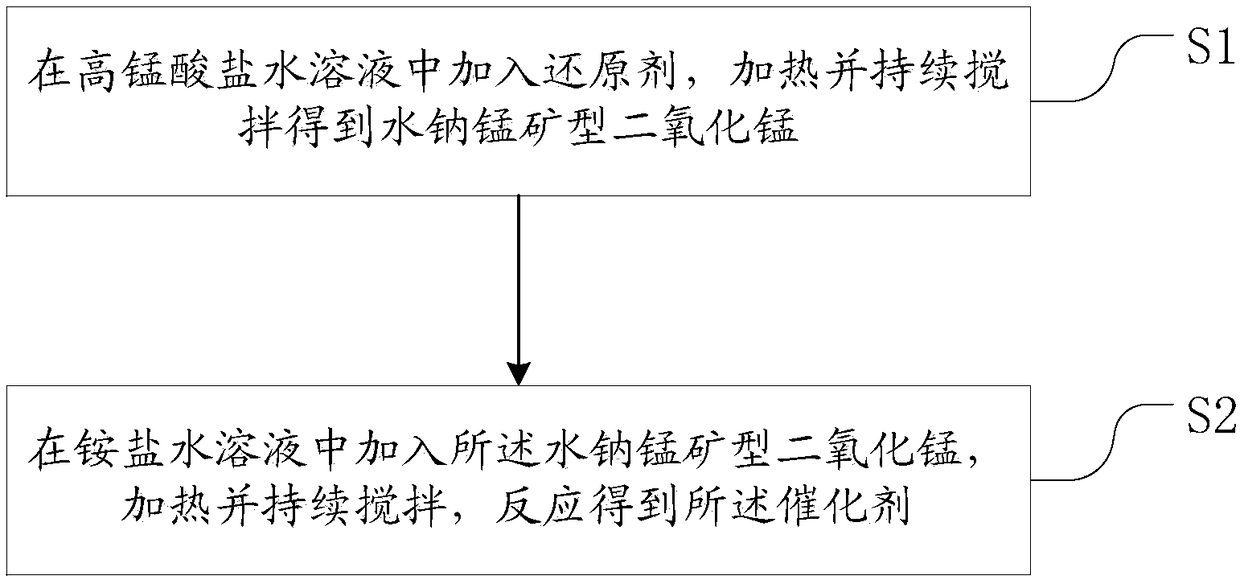
Preparation method of catalyst for ozone decomposition
ActiveCN108579729ALarge specific surface areaEnhanced Surface AcidityGas treatmentDispersed particle separationBirnessiteDecomposition
The invention provides a preparation method of a catalyst for ozone decomposition. The preparation method comprises the following steps: S1, adding a reducing agent into permanganate water solution, heating, and continuously stirring to obtain birnessite manganese dioxide; and S2, adding the birnessite manganese dioxide into an ammonium salt water solution, heating and continuously stirring to obtain the catalyst.
Owner:TSINGHUA UNIV
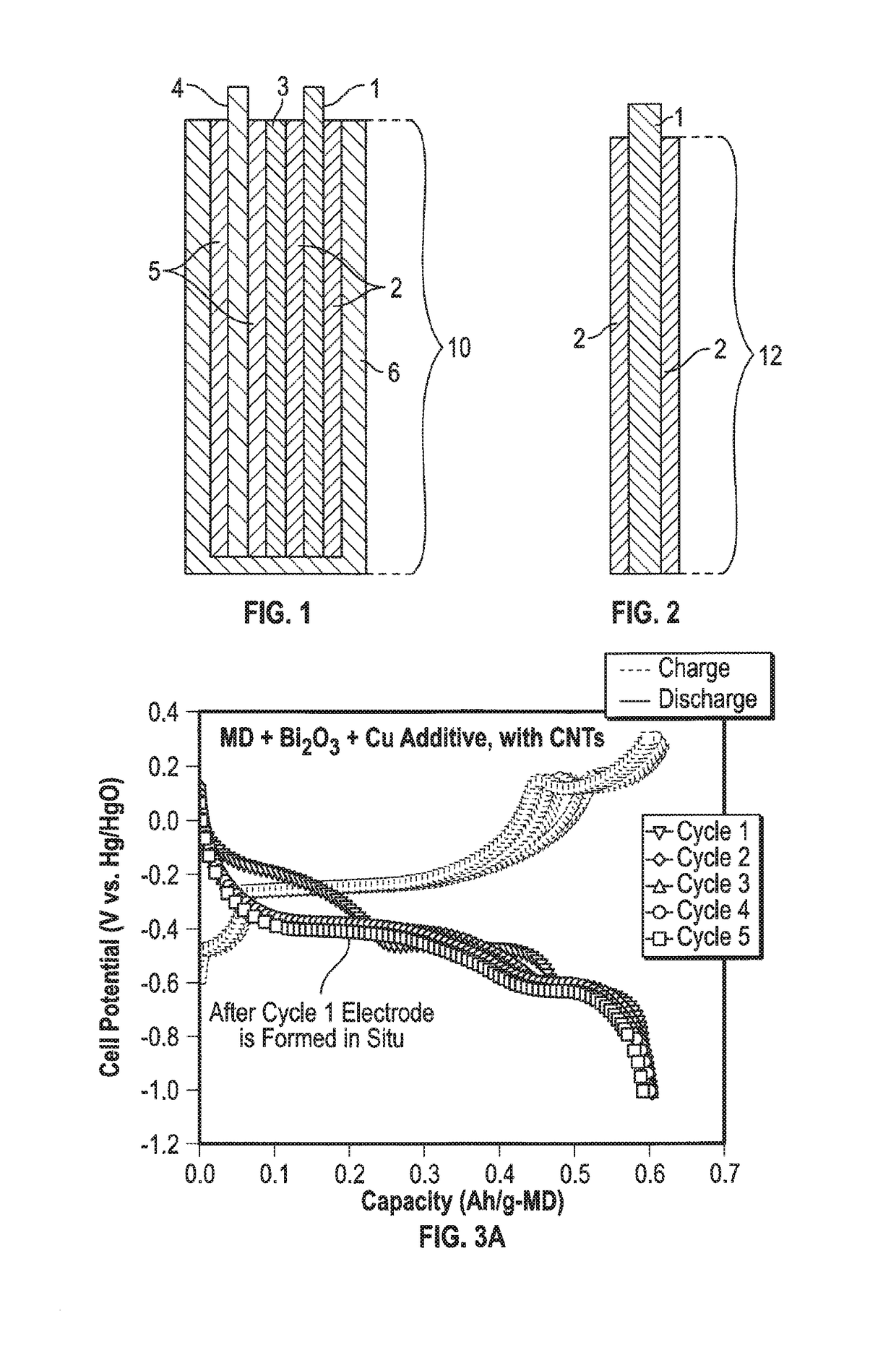
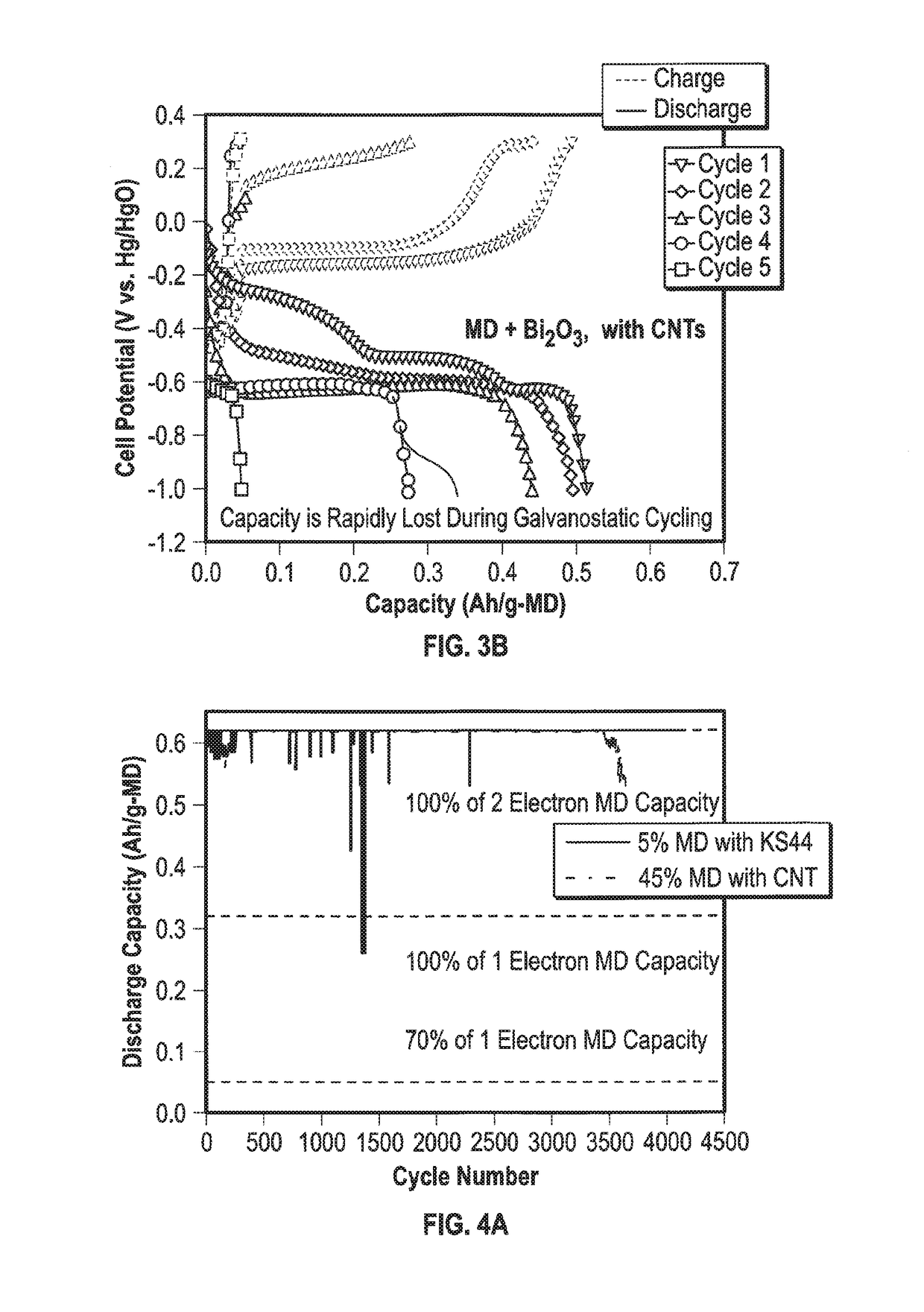
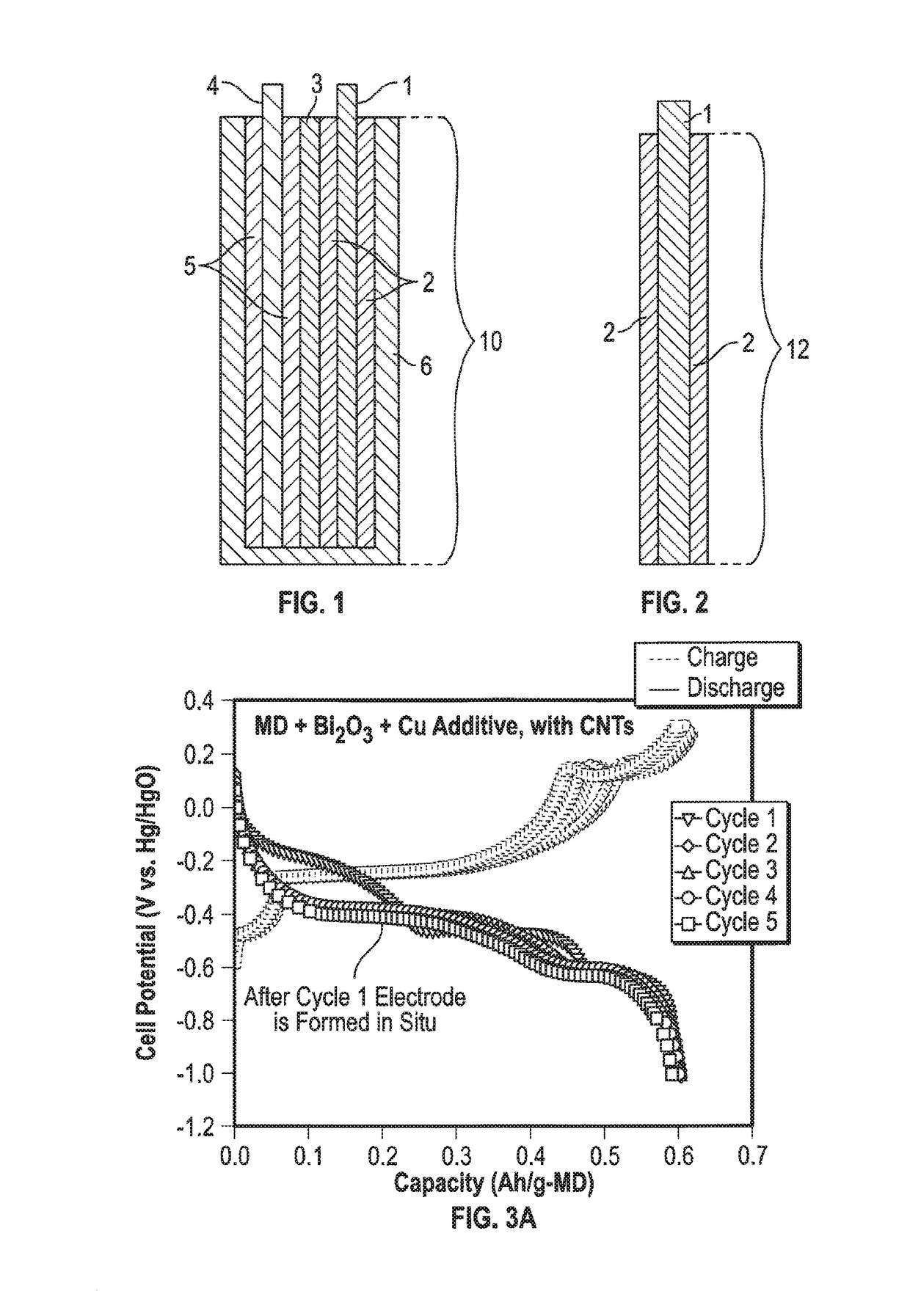
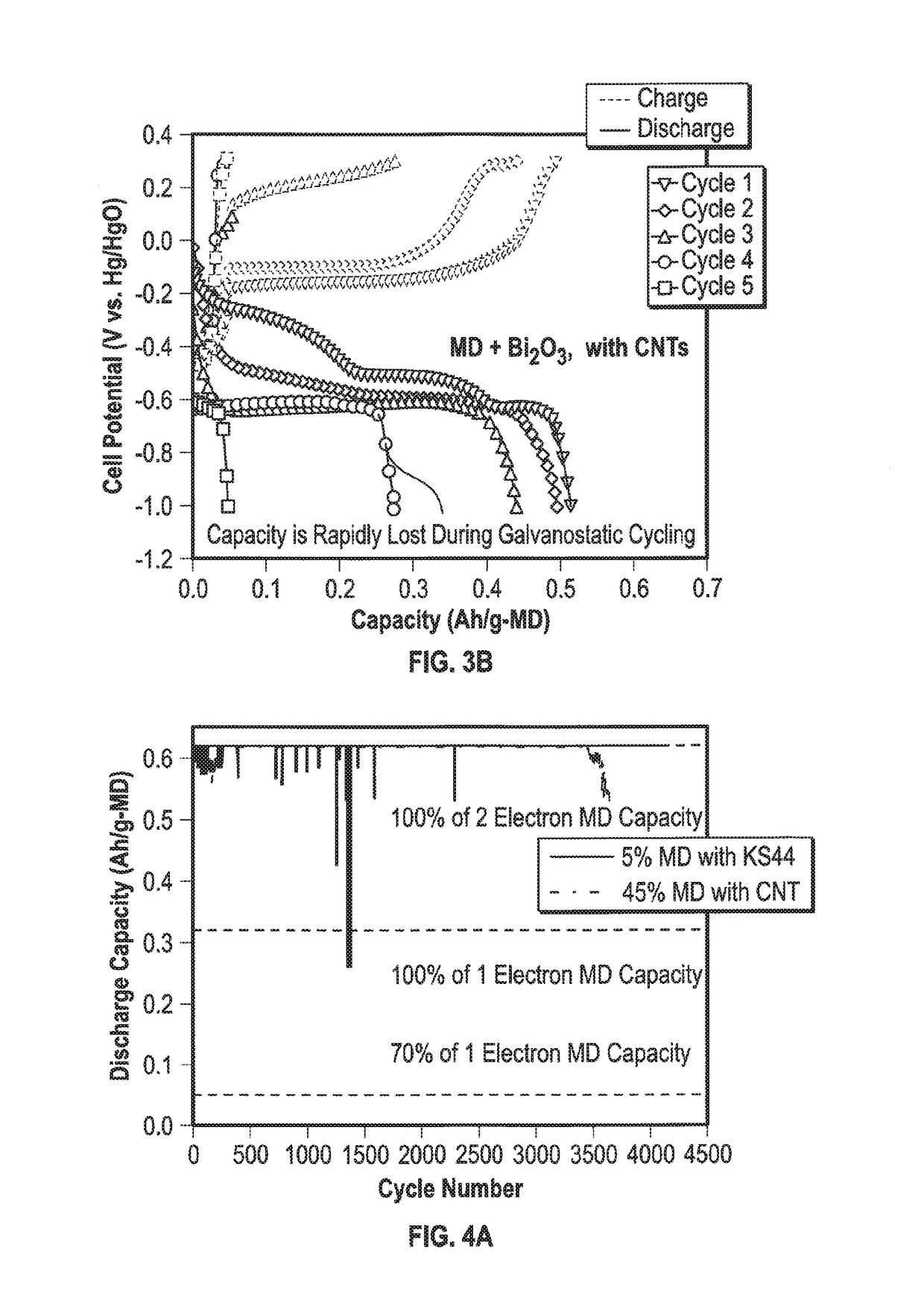
Mixed Material Cathode for Secondary Alkaline Batteries
A secondary alkaline battery using manganese dioxide is described. The battery includes a mixed cathode material with birnessite-phase manganese dioxide or electrolytic manganese dioxide (EMD), a bismuth compound and a copper compound selected from the group consisting of elemental copper and a copper salt. In some embodiments, a conductive carbon and / or a binder may also be included.
Owner:RES FOUND THE CITY UNIV OF NEW YORK
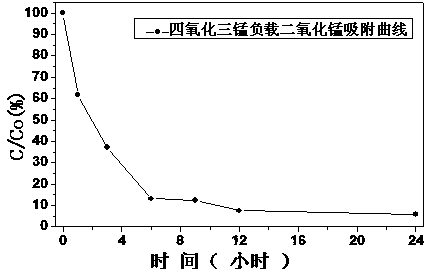
Supported manganese dioxide adsorbent and method for treating aniline waste water through same
ActiveCN103785345ALow costIncrease surface areaOther chemical processesWater contaminantsBirnessiteSorbent
The invention relates to a supported manganese dioxide adsorbent and a method for treating aniline waste water through the same, and belongs to the technical field of waste water treatment. The absorbent comprises nanometer sheet-shaped birnessite type manganese dioxide and transition metallic oxide carriers, wherein the particle size of the carriers is one hundred to thirty hundred nanometers, the thickness of the sheet-shaped manganese dioxide is one to one hundred nanometers and the content of the sheet-shaped manganese dioxide is, by weight, fifteen to twenty percent. The method for treating the aniline waste water through the supported manganese dioxide adsorbent is characterized by comprising the steps that the supported manganese dioxide adsorbent is prepared through the hydrothermal method, the supported manganese dioxide adsorbent is added into the aniline waste water with the PH value being three to fourteen, vibration is conducted fully and centrifugal separation is conducted. The adsorbent is high in adsorption capacity, not prone to agglomeration, high in adsorption efficiency, capable of being recycled, and capable of lowering the concentration of the high-concentration aniline waste water to five to seven hundred milligram per liter. The method has the advantages that operation is simple, recovery treatment is convenient and raw materials are cheap and easy to obtain.
Owner:WUHAN UNIV
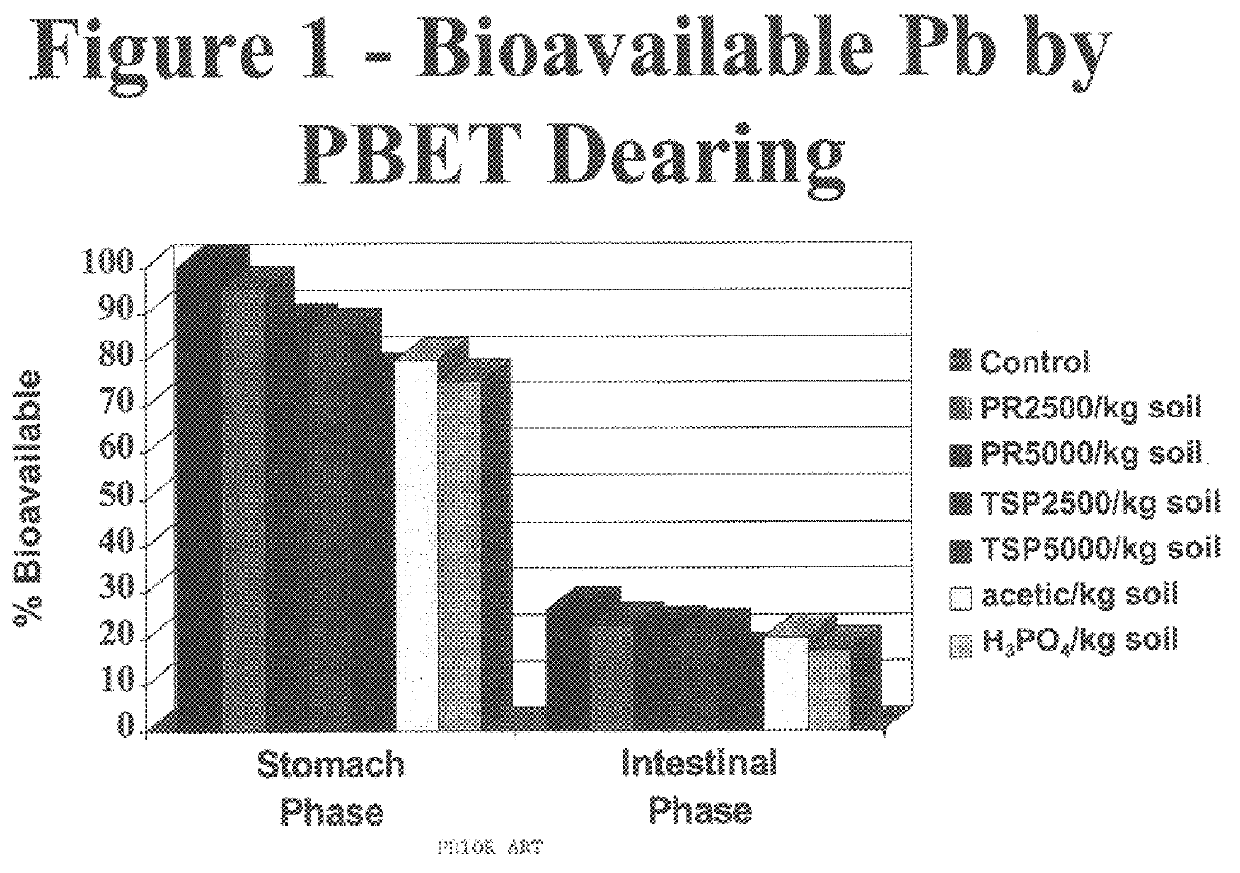
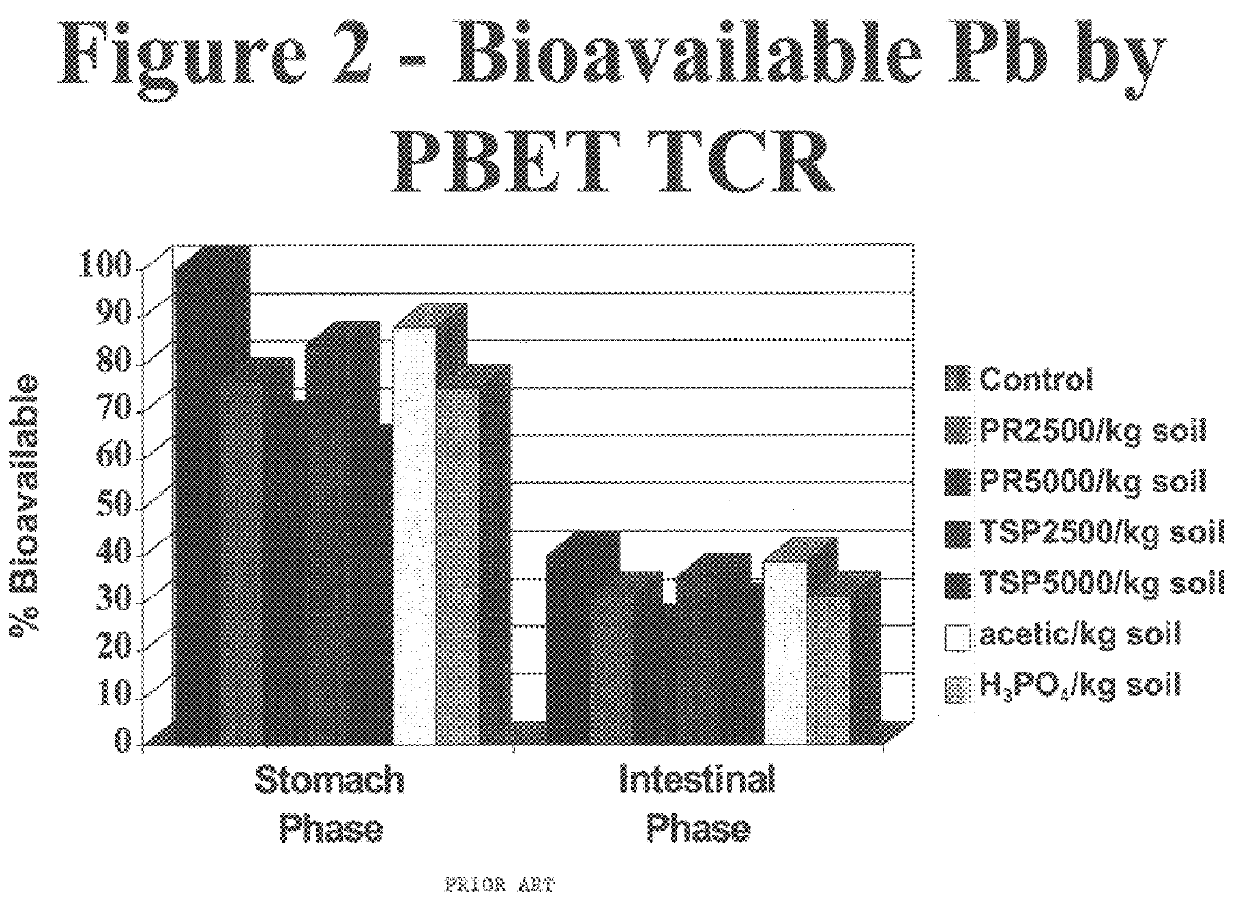
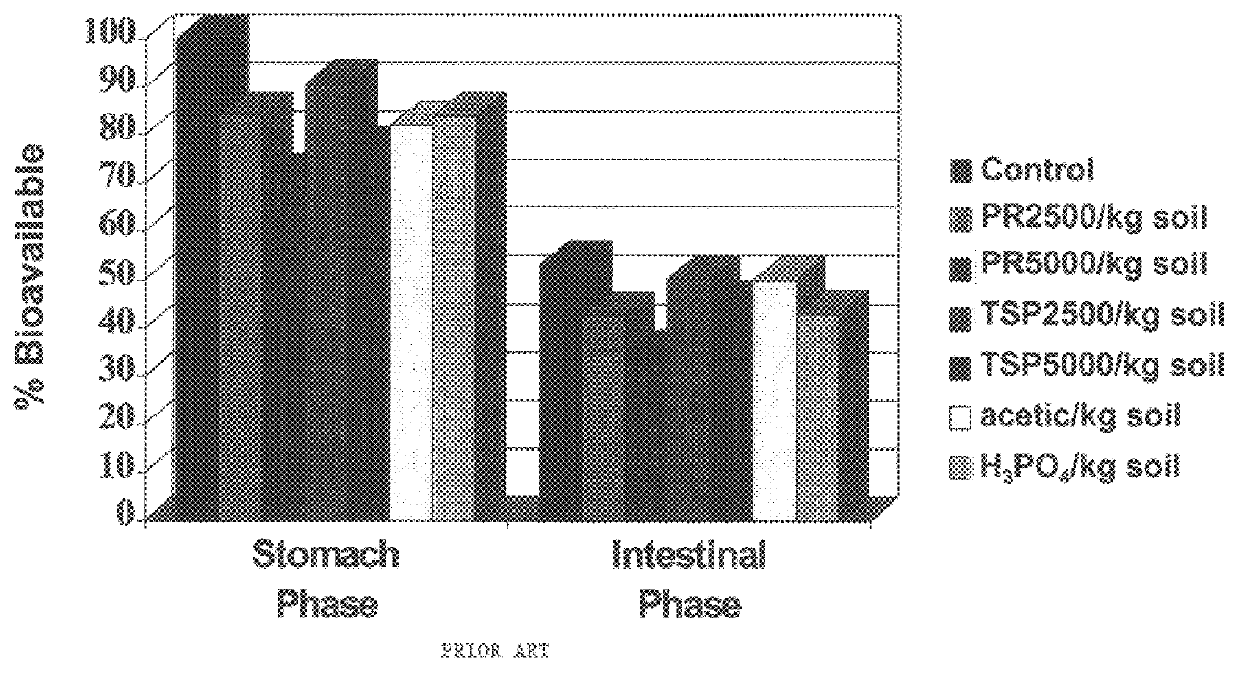
Method of in situ immobilization and reduction of metal bioavailability in contaminated soils, sediments, and wastes
InactiveUS6383128B1Reduced bioavailabilityNon-bioavailableSolid waste disposalContaminated soil reclamationContaminated soilsBirnessite
Improved methods and compositions for decreasing the bioavailability of metals in soil are provided. Broadly, the methods comprise mixing a source of phosphorus and an oxide of manganese with the contaminated soil so as to reduce the metal bioavailability in the soil. The phosphorus source and oxide of manganese can be individually mixed with the soil, or can be provided as a premix powder or granule to be mixed with the contaminated soil. Preferably, the pH of the soil is then adjusted to, and maintained at, a level of at least about 7.0. Preferred phosphorus sources include phosphate rock, alkali and alkaline earth metal phosphates, ammonium phosphates, ammonium orthophosphates, orthophosphoric acid, and superphosphates. Preferred oxides of manganese include MnO2, Mn3O4, birnessite, cryptomelane, and psilomelanes.
Owner:KANSAS STATE UNIV RES FOUND
Preparation method of high-purity birnessite type manganese oxide capable of efficiently degrading organic dyes
InactiveCN107021525ANo pollution in the processRaw materials are cheap and easy to getWater contaminantsManganese oxides/hydroxidesOrganic dyePersulfate
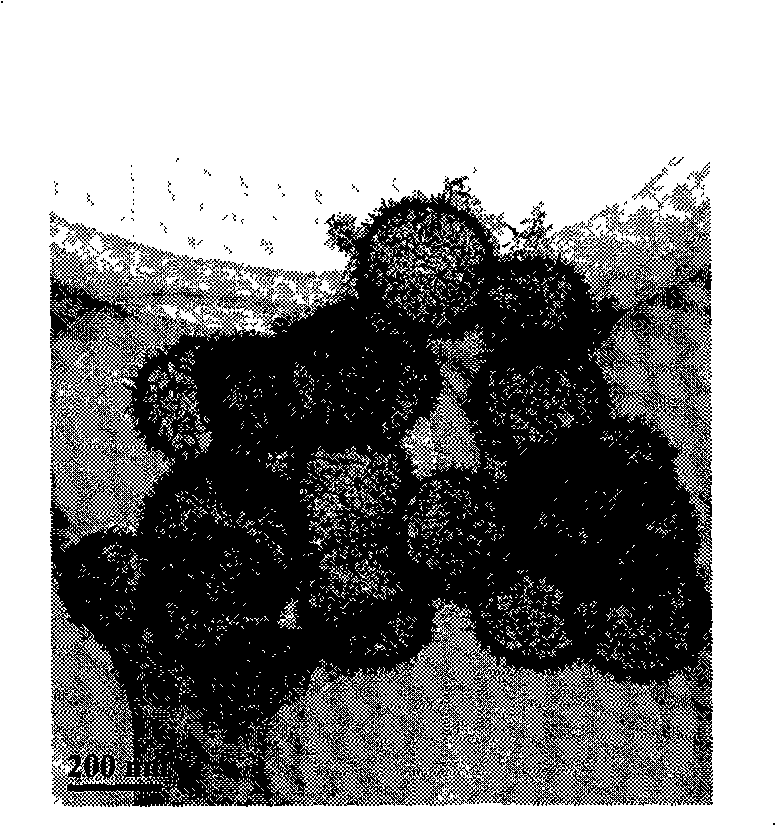
The present invention discloses a preparation method of high-purity birnessite capable of efficiently degrading organic dyes and having a three-dimensional micro-flower structure. According to the preparation method, a soluble manganese salt is adopted as a manganese source, a proper amount of a complexing agent is added to the manganese salt solution, and the high-purity birnessite having the three-dimensional micro-flower structure is obtained under an alkaline condition by using an air oxidation method. According to the present invention, the experiment results of the degradation of organic dyes such as methylene blue (MB), methyl orange (MO) and Rhodamine B (RhB) with the prepared birnessite show that the organic dyes can be efficiently degraded under the acidic condition in the absence of any oxidizing agents such as hydrogen peroxide and persulfate without any auxiliary equipment such as ultrasound, microwave and the like; the preparation method has characteristics of cheap and easily available raw material, simple preparation process, time saving, and labor saving; and the prepared product can degrade the organic dyes under the simple condition, can provide the significant effect, and can achieve the treatment on the industrial organic dye pollution.
Owner:HUNAN UNIV
Birnessite manganese oxide powder with ultrahigh specific capacitance characteristic as well as preparation method and application thereof
ActiveCN104355334ASave raw materialsIncrease productivityManganese oxides/hydroxidesCapacitanceIon exchange
The invention discloses a birnessite manganese oxide powder with ultrahigh specific capacitance characteristic as well as a preparation method and application thereof. The manganese oxide powder has an ideal chemical formula of M[2x]MnO[2+x], and in the formula, M is any combination of Li, Na and K positive ions and x is 0.1-0.5. The preparation method comprises the following steps: controlling the mole ratio of permanganate and organic fuel, and dropwise adding an organic fuel solution into a permanganate aqueous solution; heating in a muffle furnace to obtain the required birnessite manganese oxide powder. According to the birnessite manganese oxide powder disclosed by the invention, production raw materials are low in price, equipment is simple, the production efficiency is high, and the specific capacitance characteristic is excellent (the specific capacitance can reach 1,055F.g<-1> under the current density of 1A.g<-1>); the birnessite manganese oxide powder can be applied to the fields of supercapacitors, lithium / sodium / magnesium ion batteries, ion exchange, water photolysis and the like, and is wide in application range.
Owner:TAIYUAN UNIV OF TECH
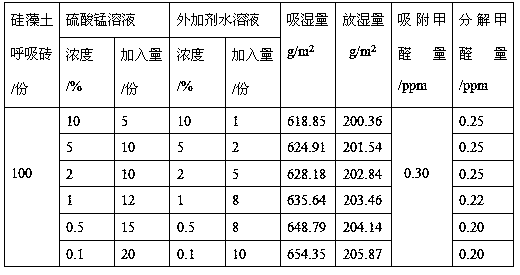
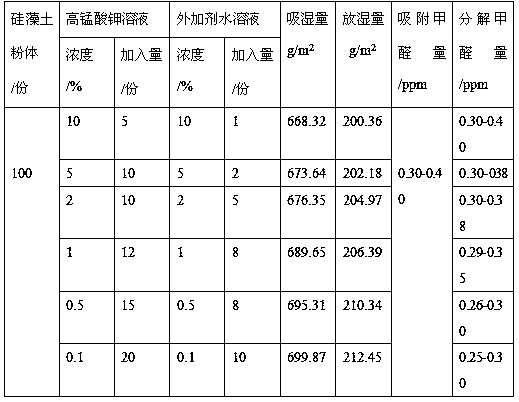
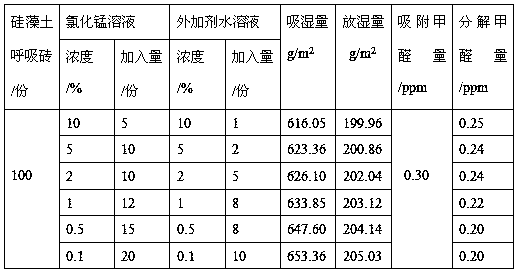
Preparation method for diatomite loaded birnessite type manganese dioxide
The invention discloses a preparation method for diatomite loaded birnessite type manganese dioxide, and relates to a preparation method for manganese dioxide. The preparation method is characterizedin that a manganese salt and an additive are prepared into a uniform aqueous solution; diatomite and a product thereof are soaked into an aqueous solution of the manganese salt and the additive, and drying and / or forming treatment is performed; the diatomite loaded birnessite type manganese dioxide is prepared from 5-20 parts of a manganese salt solution, 1-10 parts of an additive aqueous solutionand 100 parts of diatomite and a product thereof, wherein manganese salt is prepared from potassium permanganate, manganese chloride and manganese sulfate, and concentration of the manganese salt is0.1-10.0%; concentration of the additive aqueous solution is 0.1-10.0%; and the diatomite and the product thereof are prepared from diatomite powder and a diatomite breathing brick; the loaded birnessite type manganese dioxide diatomite is prepared. Birnessite type manganese dioxide is loaded onto the diatomite and the product thereof, and moisture absorption of diatomite and formaldehyde adsorption are organically combined with formaldehyde decomposition and regeneration of birnessite type manganese dioxide, so that indoor air is efficiently purified.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method for preparing potassium ion battery electrode potassium type birnessite
The invention discloses a method for preparing a potassium type birnessite manganese ore layered crystal potassium ion battery electrode material from a potassium salt and tetravalence manganite witha high-temperature solid-phase method together with an ion exchange method process. Due to adoption of the ion exchange process, the content of potassium ions in potassium type birnessite manganese ore can be increased, and properties of a potassium ion battery electrode are improved. The high-potassium type birnessite electrode material of water solution exchange is high in electric property, thecurrent density of the material is 16mA*g<-1>, the mass specific capacity of the material is up to 125mAh*g<-1>, and when the current density of the material is increased to 400mA*g<-1>, the mass specific capacity of the material is still maintained at 68mAh*g<-1>. After 50 times of circulation at a current density of 80mA*g<-1>, the mass specific capacity of the material is still maintained at 68mAh*g<-1>, and the capacity retention rate of the material is 79%. When being used as a potassium ion battery electrode material, a sample prepared with the method is excellent in rate capability andcirculation stability.
Owner:HANGZHOU INST OF ADVANCED MATERIAL BEIJING UNIV OF CHEM TECH
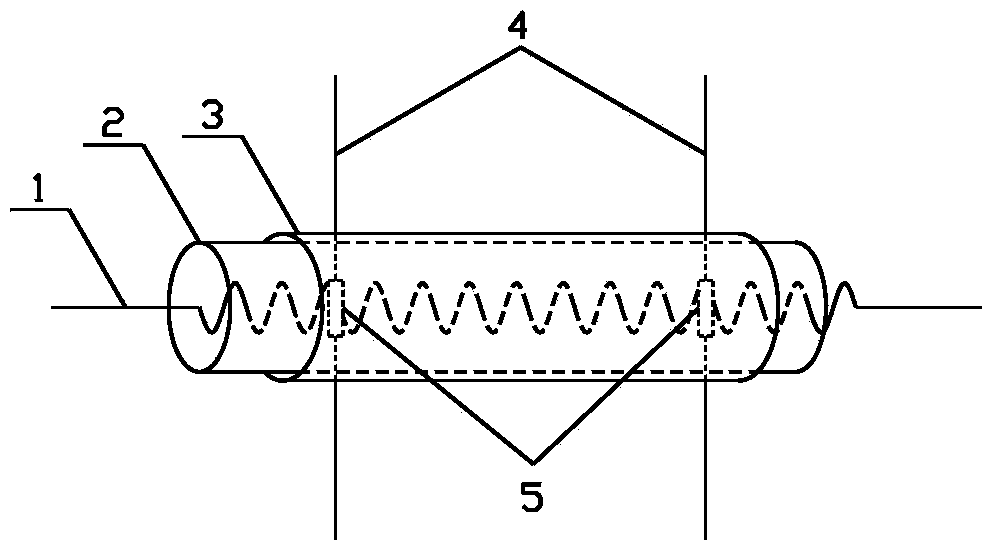
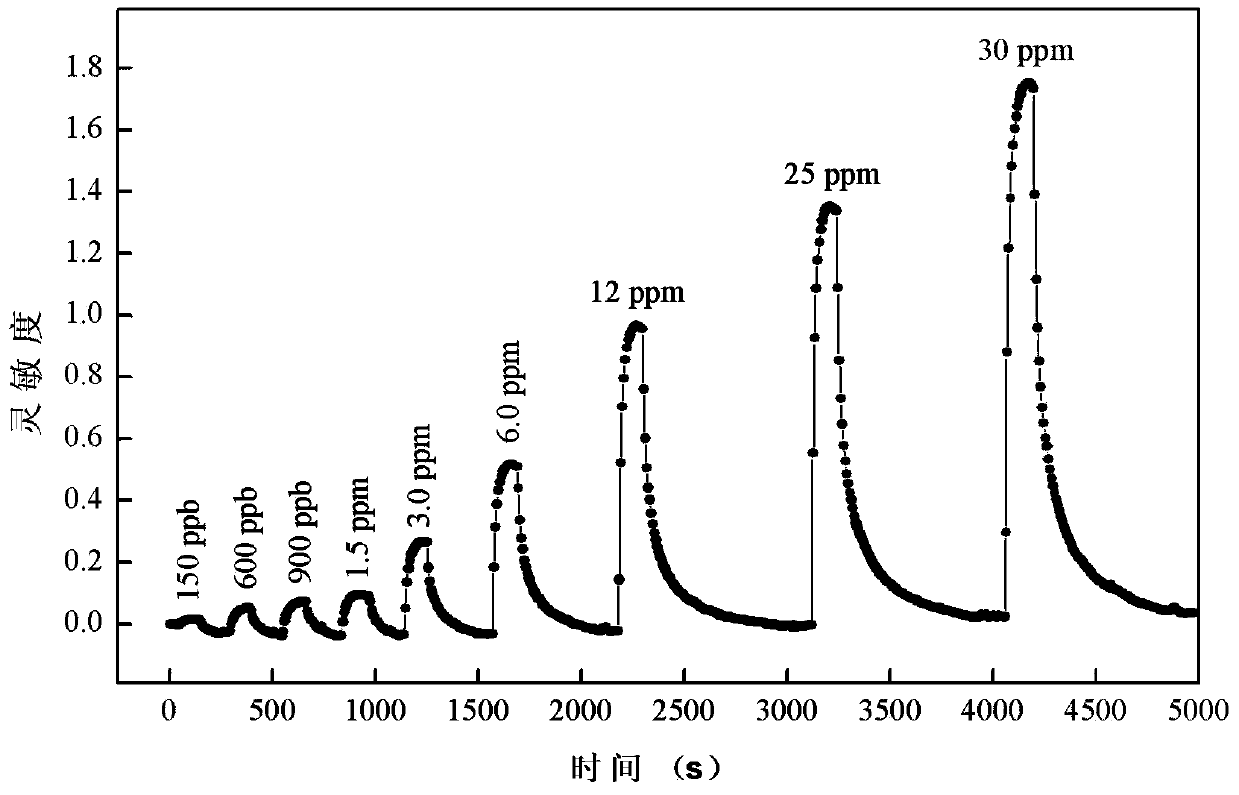
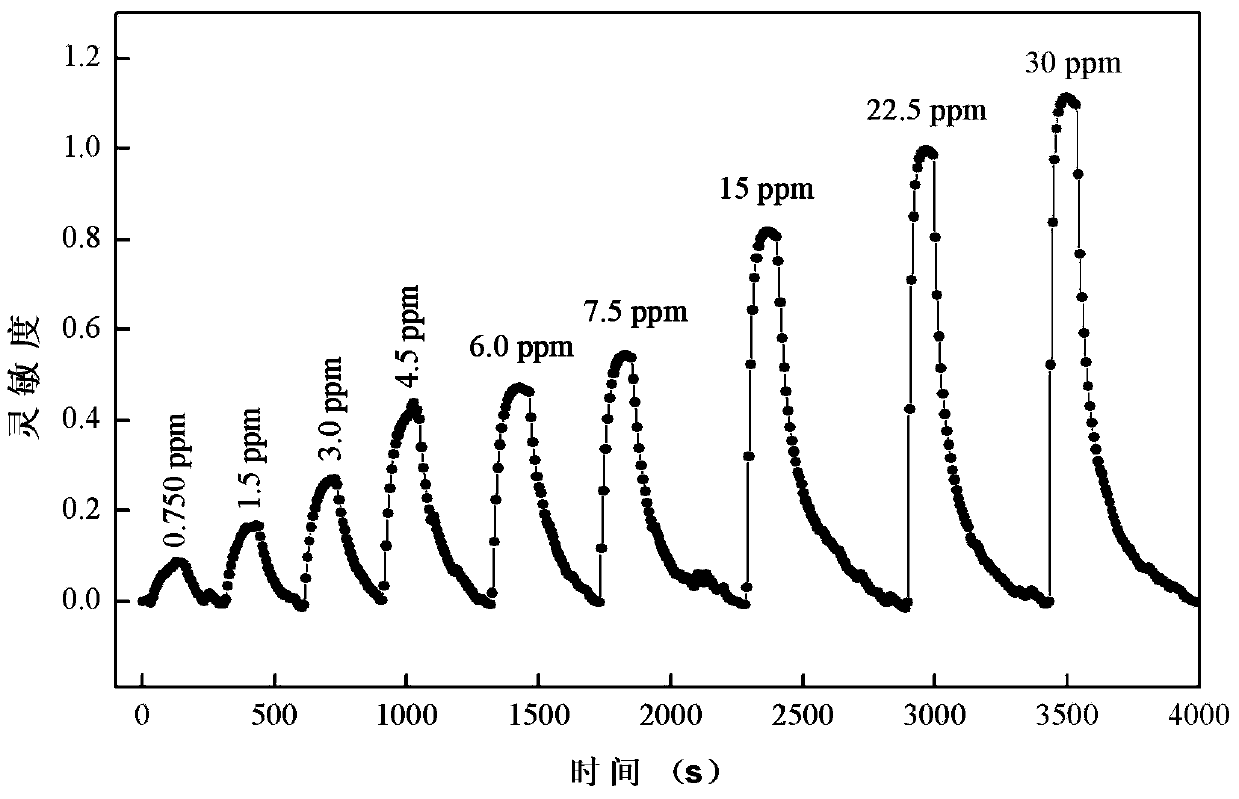
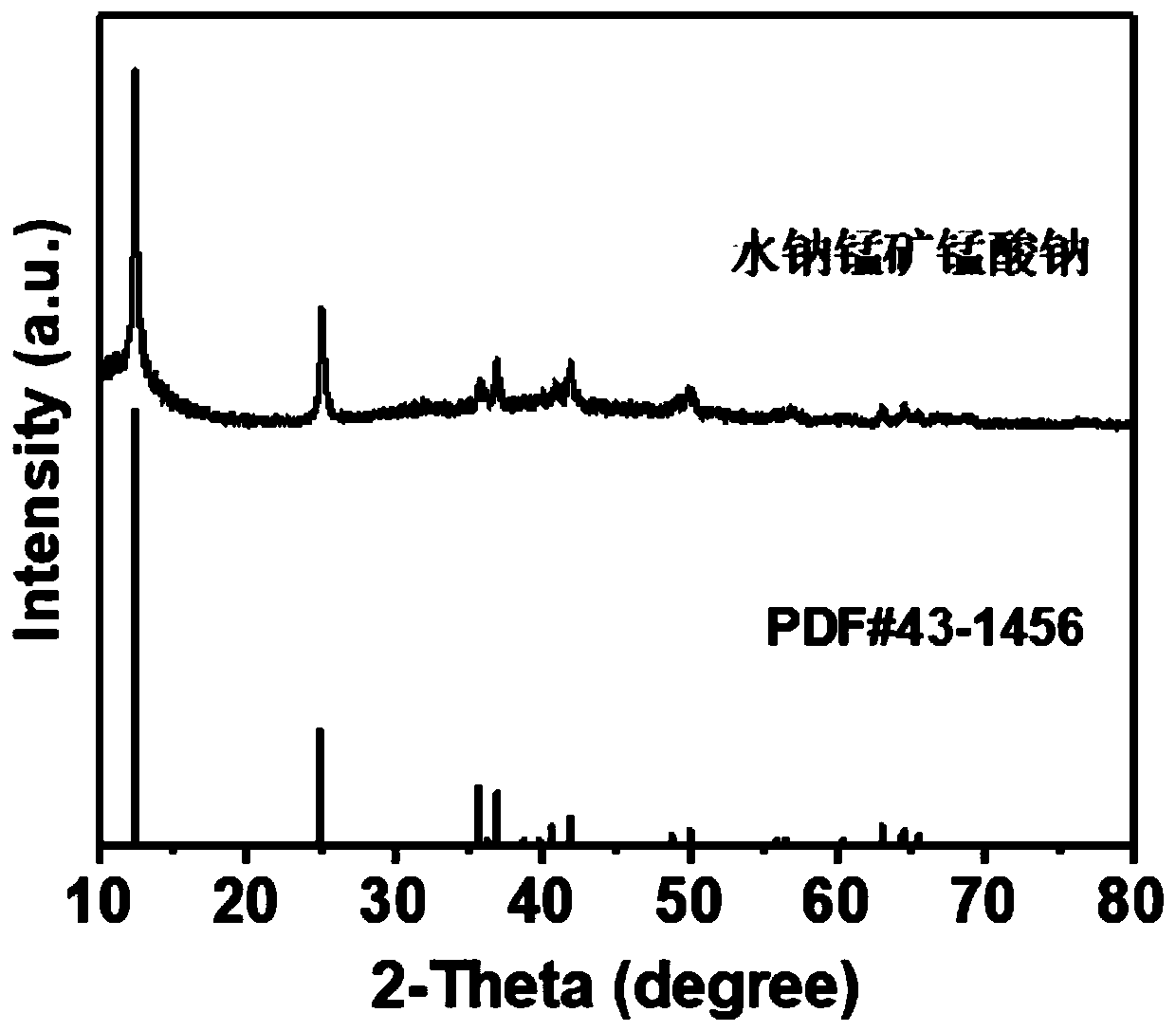
Birnessite type manganese dioxide nanosheet hydrogen sensor and preparation method thereof
The invention discloses a birnessite type manganese dioxide nanosheet hydrogen sensor and a preparation method thereof. The preparation method comprises the following steps: (1) preparing birnessite type manganese dioxide; (2) preparing an indirectly-heated hydrogen-sensitive element; (3) respectively welding a conducting wire and a resistive heater onto a sensor base to manufacture an indirectly-heated hydrogen sensor. According to the invention, a manganese acetate solution and a sodium peroxide solution are used as raw materials and a direct-oxidation coprecipitation technique is adopted to prepare a birnessite type manganese dioxide nanosheet which has the advantages of large specific area, high porosity, small grain size, high adsorption activity and poor crystallization property. The birnessite type manganese dioxide nanosheet is adopted as the hydrogen-sensitive element, so that the sensitivity of the sensor is increased, and the working temperature is reduced.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
Chargeable and dischargeable aqueous aluminum ion battery and preparation process thereof
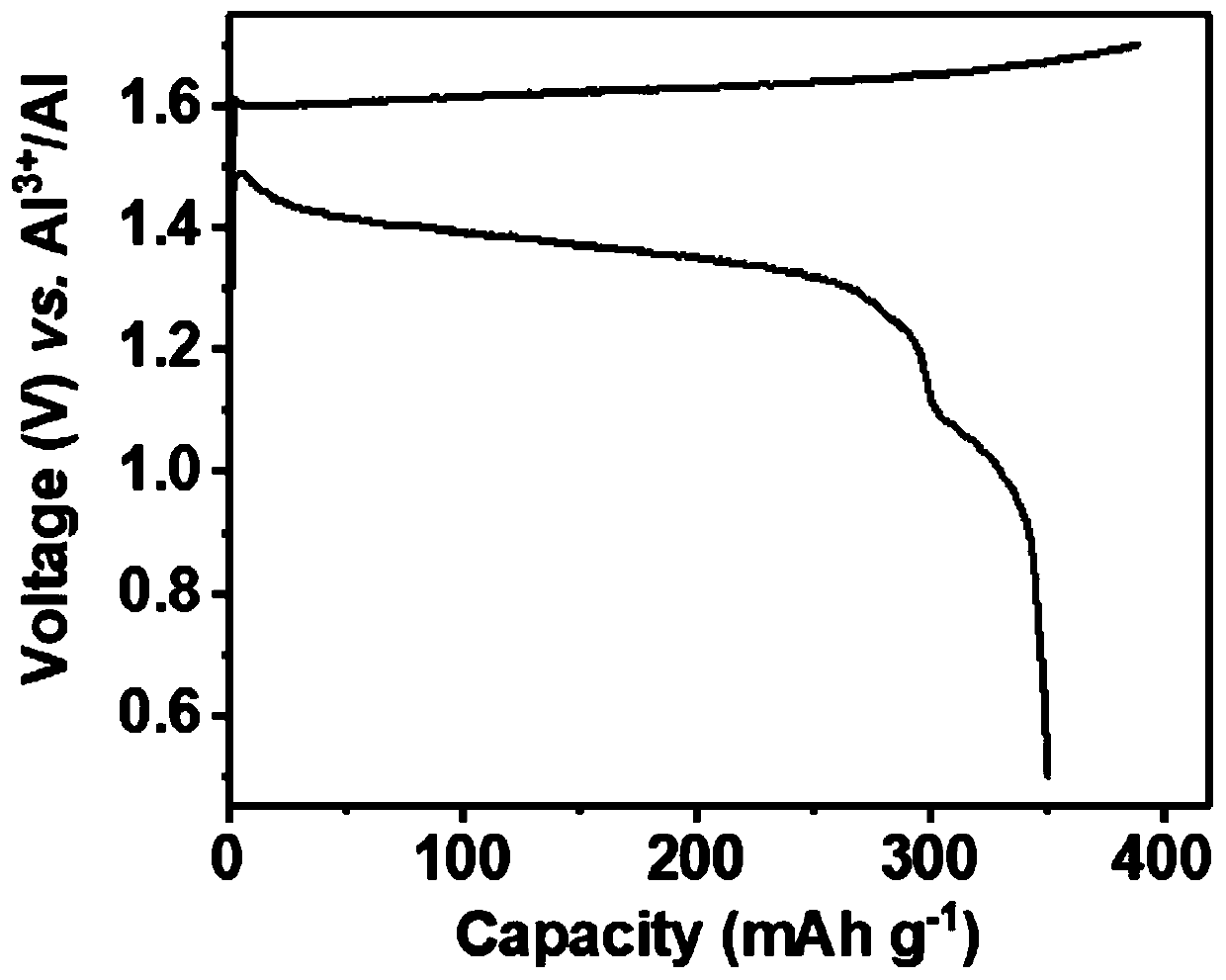
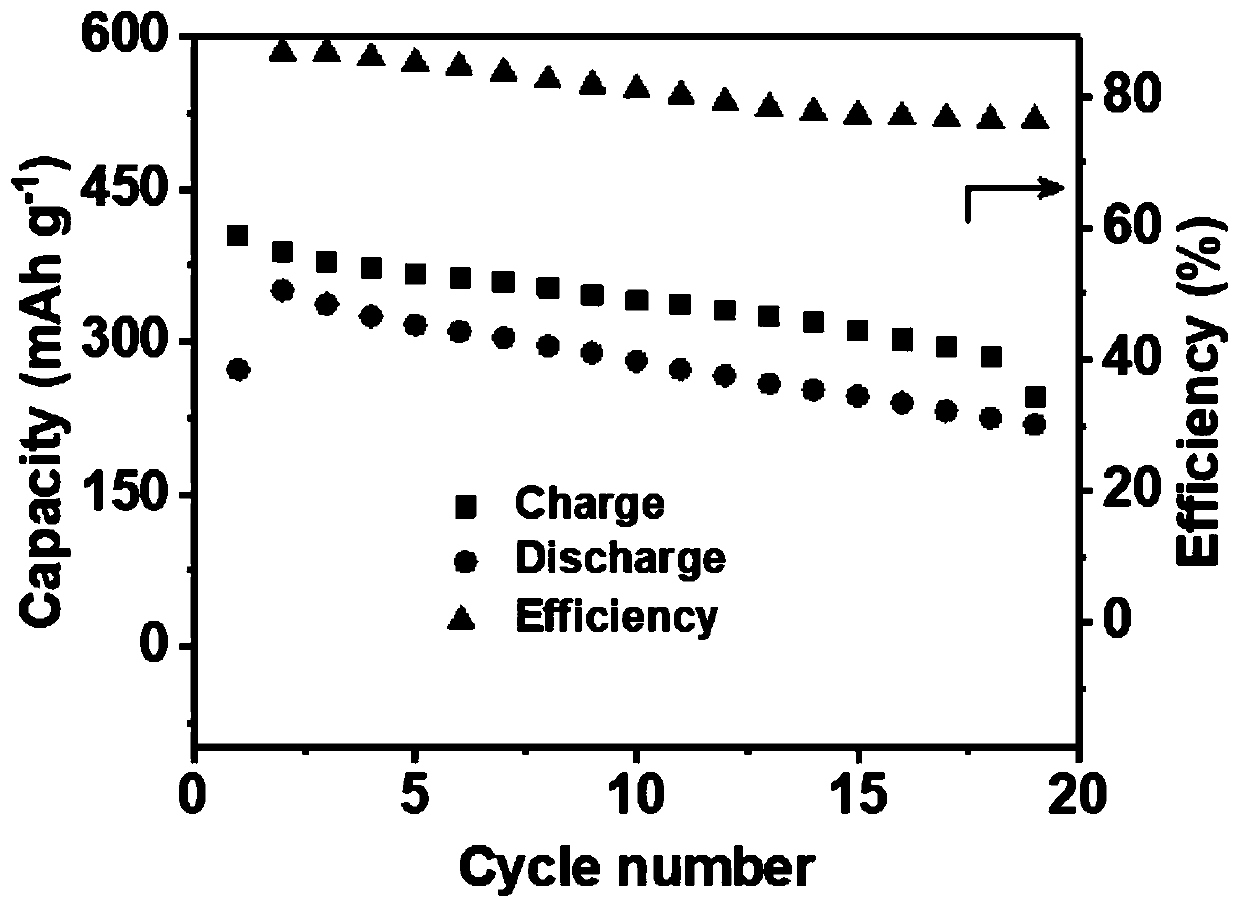
ActiveCN110010888AExcellent discharge specific capacityImprove cycle performanceSecondary cellsPositive electrodesAluminum IonBirnessite
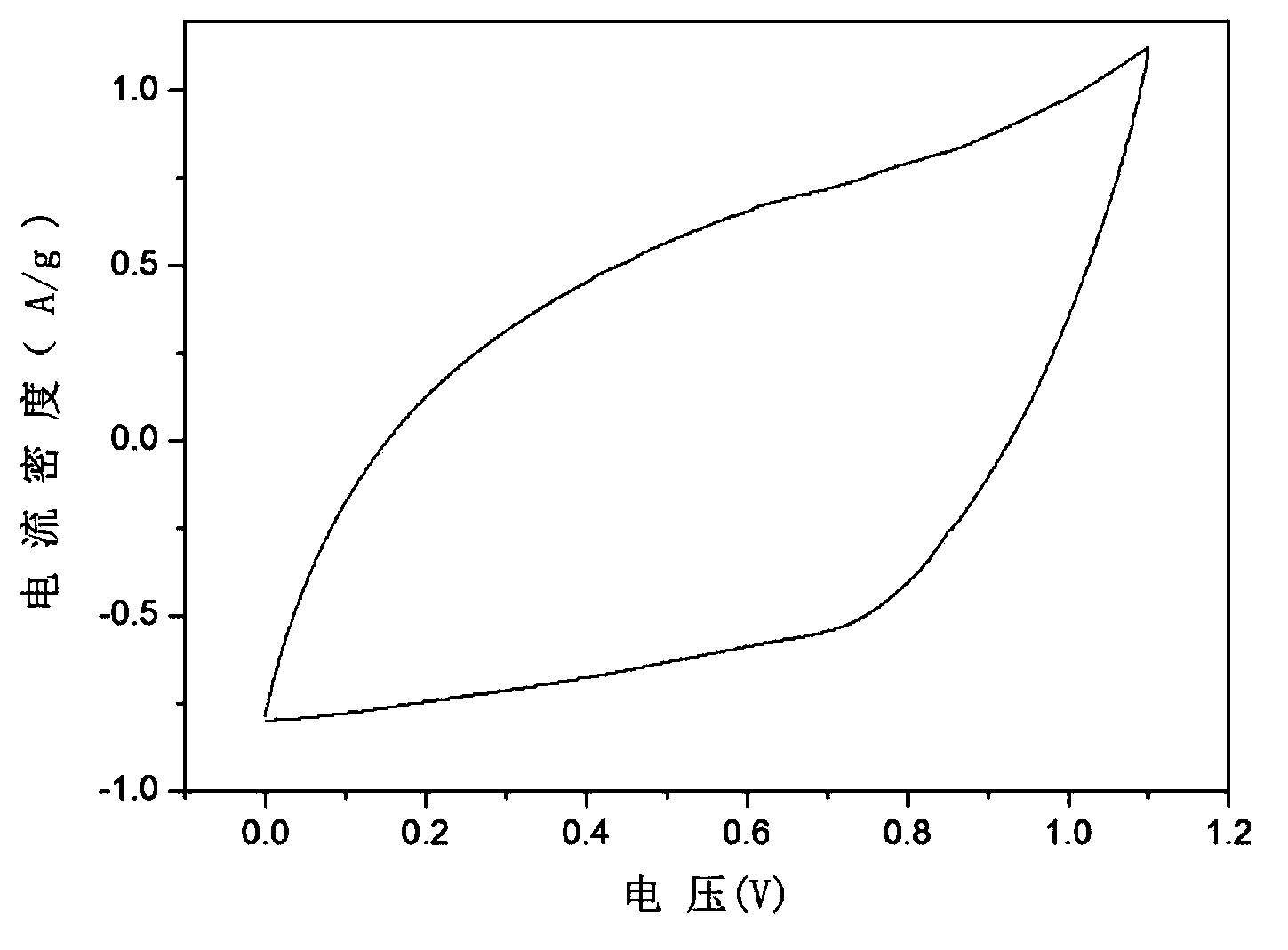
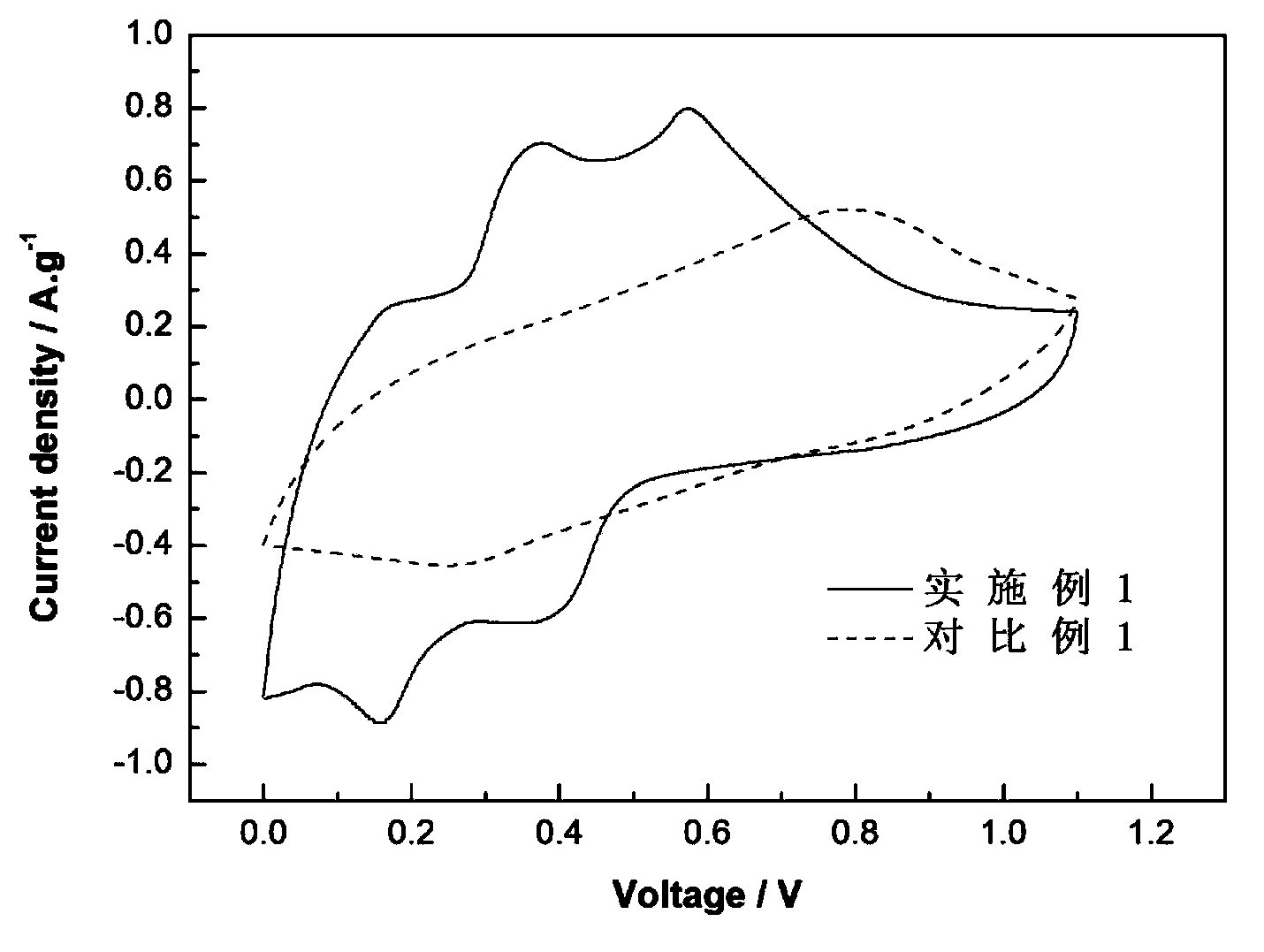
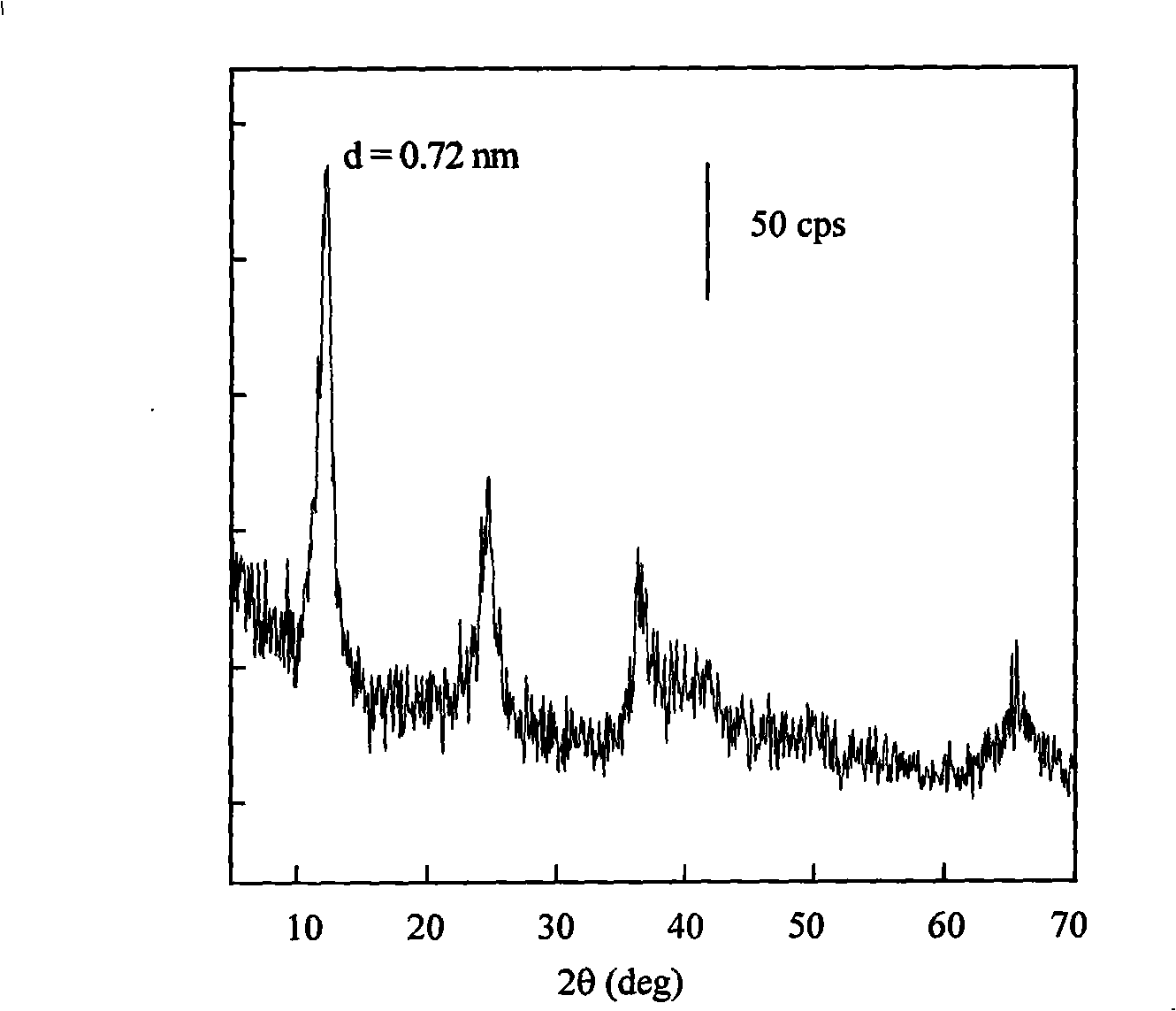
The invention relates to a chargeable and dischargeable aqueous aluminum ion battery and a preparation process thereof, belonging to the technical field of batteries. The aqueous aluminum ion batterymainly comprises a cathode, an anode, an electrolyte and a diaphragm, wherein the cathode material is birnessite manganate, the anode is metal aluminum and alloy thereof, the electrolyte is an aluminum trifluoromethanesulfonate aqueous solution, and the cathode, the anode, the electrolyte and the diaphragm form a primary battery system. The interlayer spacing of the birnessite manganate MxMnOy.nH2O (M is a metal cation) is about 0.72nm, and lattice water exists between the layers. The secondary aluminum ion battery is high in capacity (530mAhg<-1>, relative to the birnessite manganate), is proper in discharge potential platform (1.0-1.4V vs. A13+ / Al), is very good in the corresponding cathode capacity density (530-740Wh g<-1>), is wide in material source, easy to prepare, simple to assemble, low in cost and green and environmental.
Owner:BEIJING UNIV OF TECH
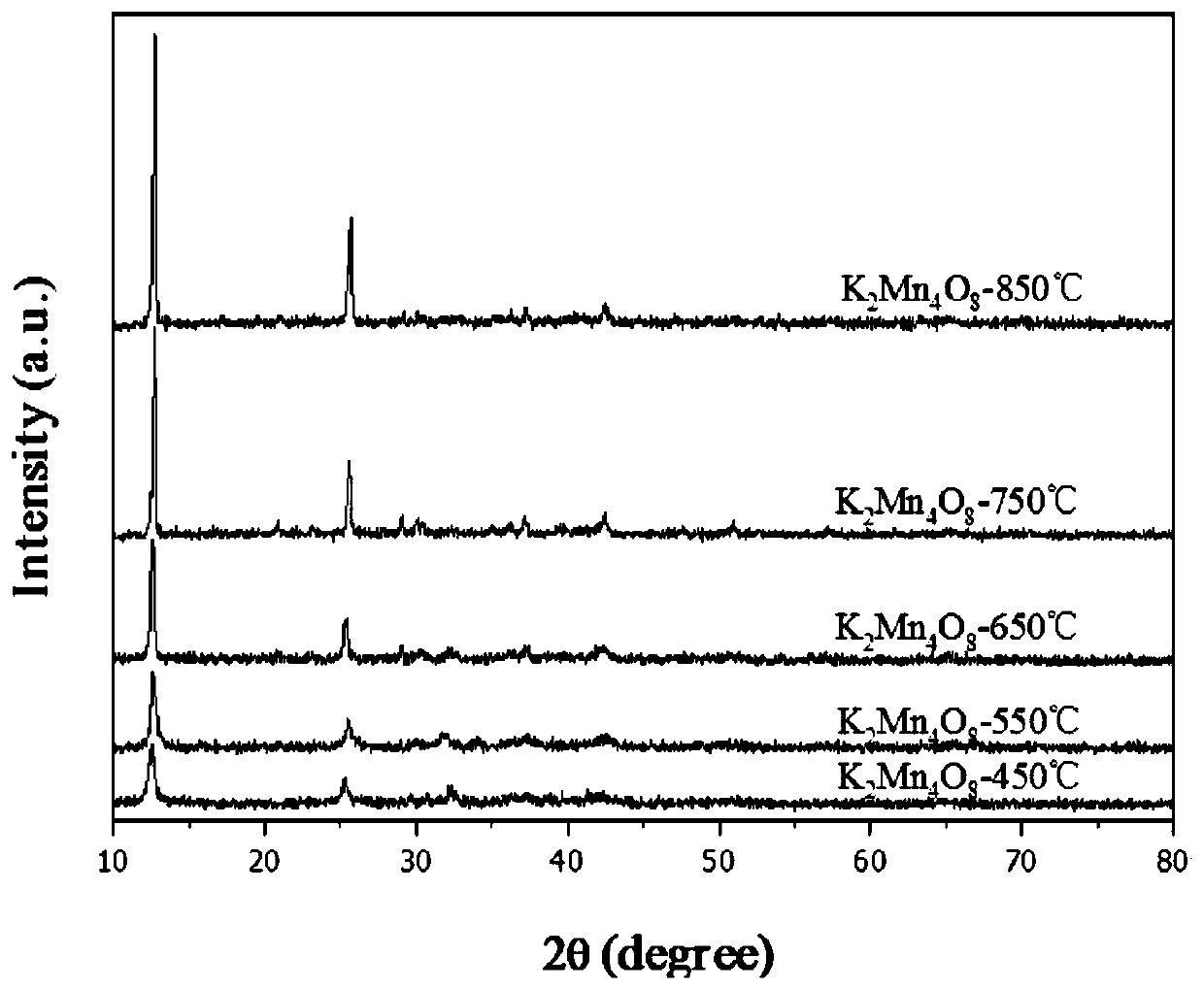
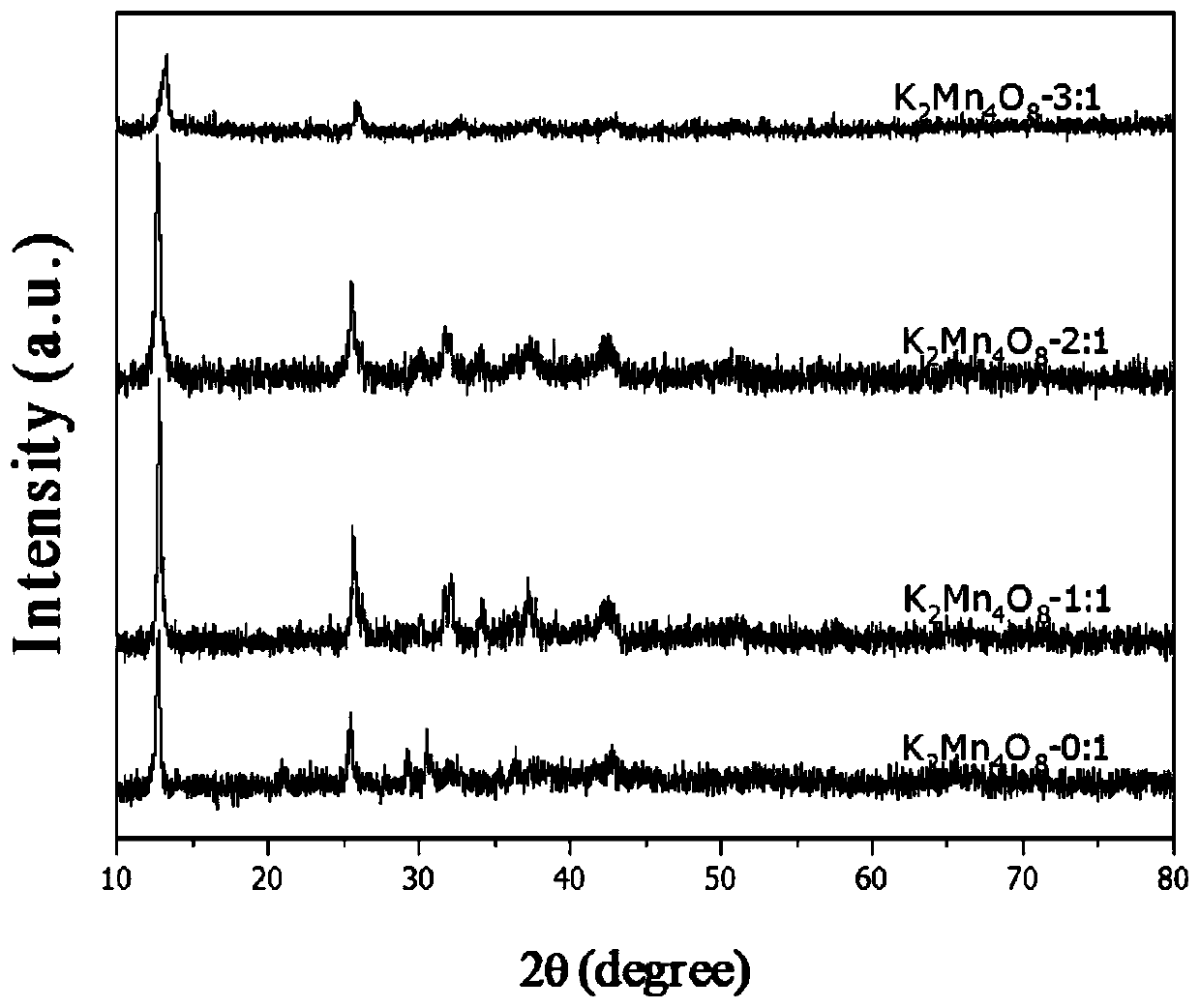
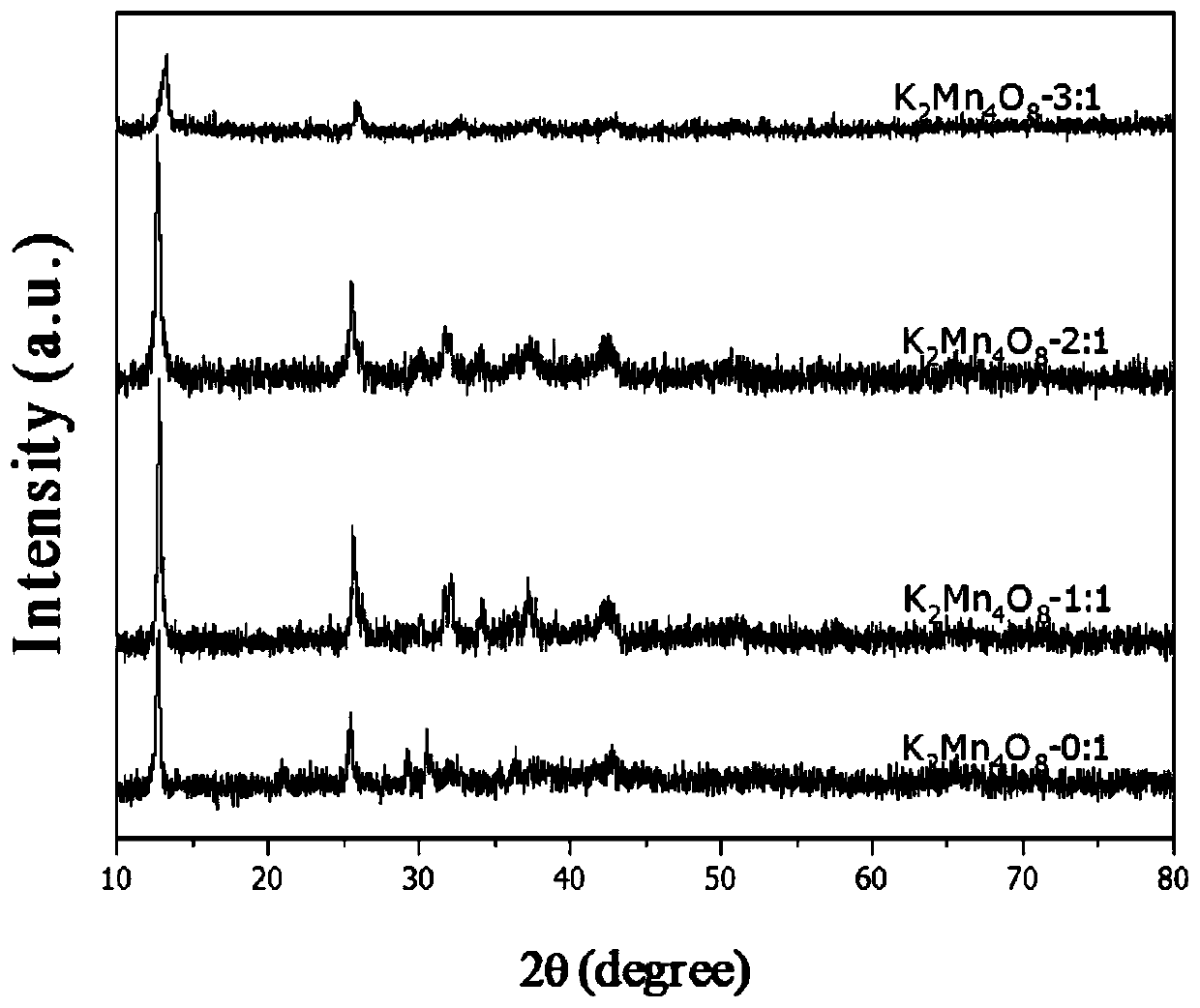
Birnessite type manganese oxide catalyst preparation method and application
InactiveCN110038555ALow costSimple preparation processExhaust apparatusSilencing apparatusBirnessiteManganese oxide
The invention relates to a preparation method of a birnessite type manganese oxide catalyst material and application thereof in charcoal smoke particle catalytic combustion. According to the birnessite type manganese oxide catalyst preparation method disclosed by the invention, the material is a manganese oxide catalyst material with a birnessite type structure, a manganese oxide material is K2Mn4O8, and the manganese oxide catalyst is a catalyst material with the molar ratio of K to Mn to O as 2 to 4 to 8. According to the manganese oxide catalyst, potassium permanganate and glucose which arelow in cost are utilized as raw materials; by means of a simple preparation method of using the glucose as a reducing agent, the birnessite type K2Mn4O8 manganese oxide catalyst with higher oxidationreduction ability is further obtained. The preparation method disclosed by the invention avoids special equipment and severe conditions, and the prepared birnessite type manganese oxide catalyst hasthe advantages of simple preparation technology, strong practicability and easiness in achieving large-scale production.
Owner:SHENYANG NORMAL UNIV
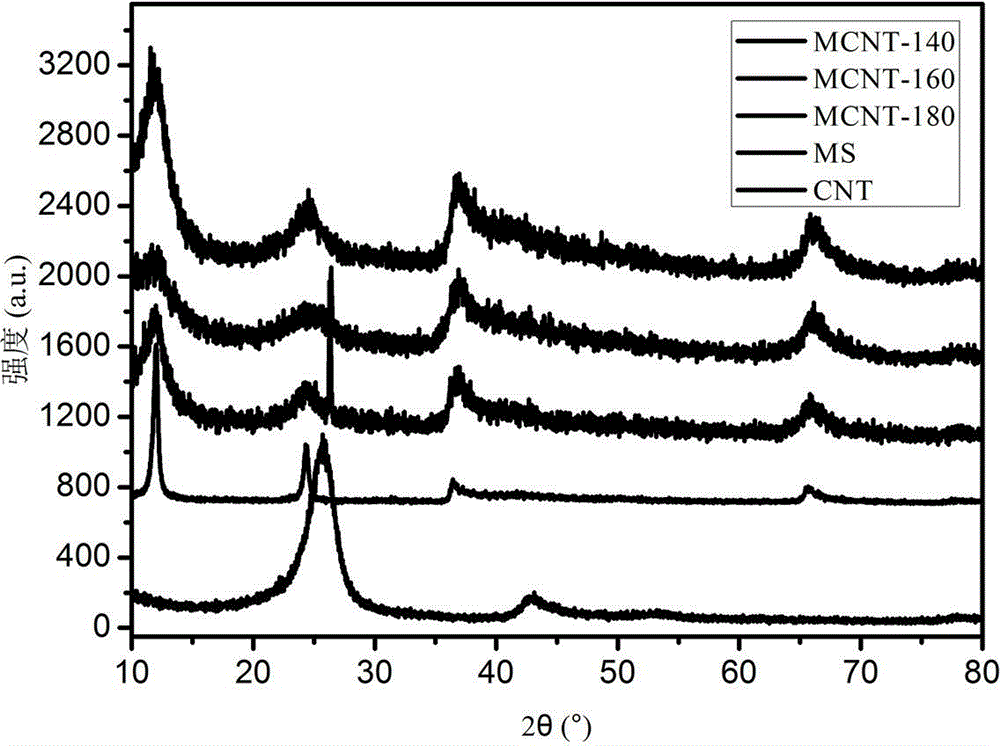
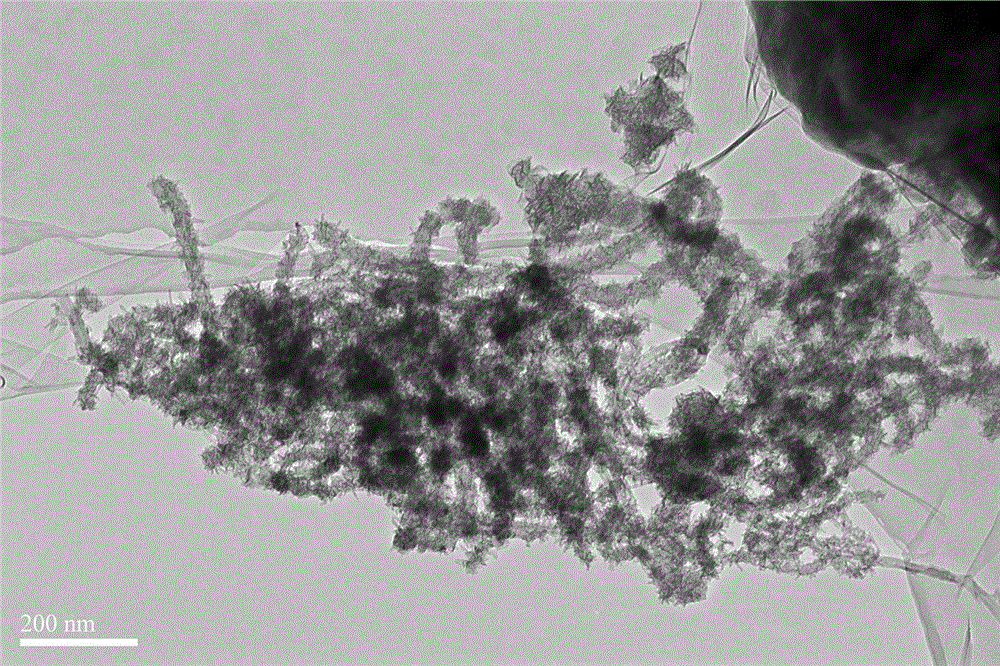
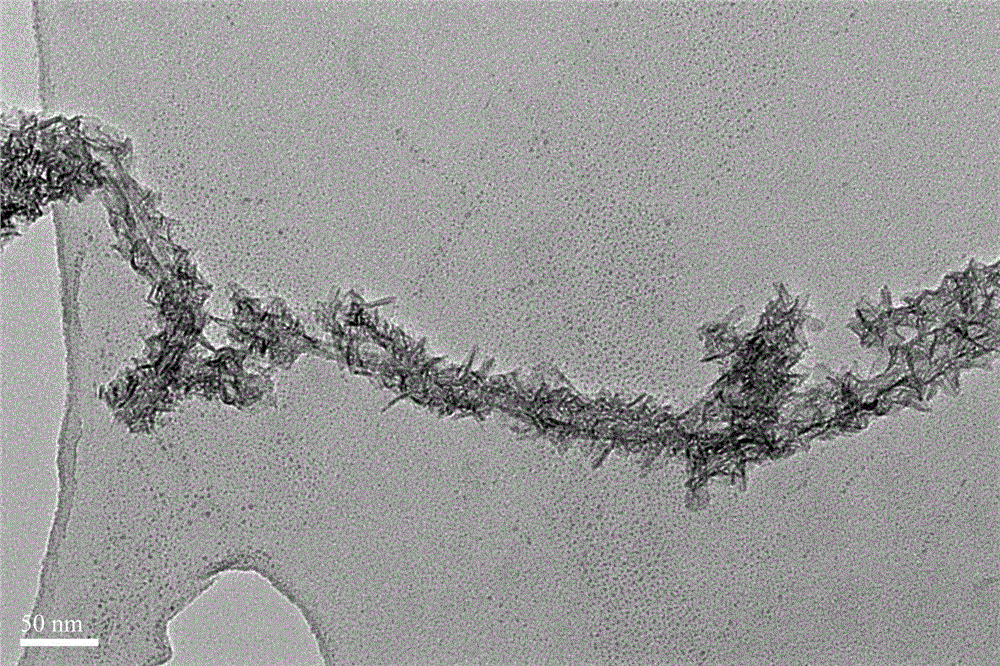
Multi-walled carbon nano-tube supported manganese oxide-based catalyst preparation method
ActiveCN104971716ALarge specific surface areaEasily exposedDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsBirnessiteParking area
The present invention relates to a multi-walled carbon nano-tube supported manganese oxide-based catalyst preparation method, wherein an active component manganese oxide is loaded on multi-walled carbon nano-tubes by using an oxidation reduction method, and the catalyst manganese-based oxide obtained after drying is loaded on the carbon nano-tubes in a scaly manner, such that the specific surface area is increased, the relatively more active sites are easily exposed compared with the loading-free birnessite type manganese oxide, and the NO catalytic oxidation reaction activity under the normal temperature and normal pressure and the long-term stability are improved; and the process is simple, and the method can be used for the efficient purification treatment of the NO pollutants in parking areas, underground shopping malls and other enclosed spaces or urban highway tunnels, and has important social significance and practical application values.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Method for treating nuclear power plant radioactive waste liquid based on birnessite in-situ reaction
ActiveCN105355250AEfficient removalReduce volumeRadioactive decontaminationLiquid wasteRadioactive waste
The invention relates to a method for treating nuclear power plant radioactive waste liquid based on the birnessite in-situ reaction. The method comprises following steps: (1) adding potassium permanganate into the nuclear power plant waste liquid containing radioactive elements and uniformly mixing the liquid; (2) adding manganous salt into the liquid on the basis that the molar ratio of manganous ions (Mn2+) in the potassium permanganate to manganous ions (Mn2+) in the manganous salt is 3:2, and uniformly mixing the liquid to obtain the mixed solution; (3) adding an alkaline solution into the mixed solution drop by drop so as to adjust the pH of the mixed solution to be 8-13, enabling the potassium permanganate to react with the manganous salt to generate birnessite MnO2, then adding magnetic Fe3O4 powder into the solution, and stirring the solution at a constant temperature of 20-80 DEG C for 15-300 minutes; and (4) allowing the solution to stand, performing magnetic separation and removing the precipitate, thereby completing the purification treatment of the nuclear power plant radioactive waste liquid. Compared with the prior art, the method is advantageous in that the steps are simple, conditions are easy to control, the method is economical and practical, the radioactive ion removing efficiency is high, the using range of the method is wide, and no secondary pollution is formed.
Owner:EAST CHINA UNIV OF SCI & TECH
Magnetite and birnessite aggregate-form mixture, synthesis method therefor, and water-treatment method using mixture
ActiveUS20130200001A1Easy to synthesizeSmall particle sizeOther chemical processesSpecific water treatment objectivesBirnessiteSynthesis methods
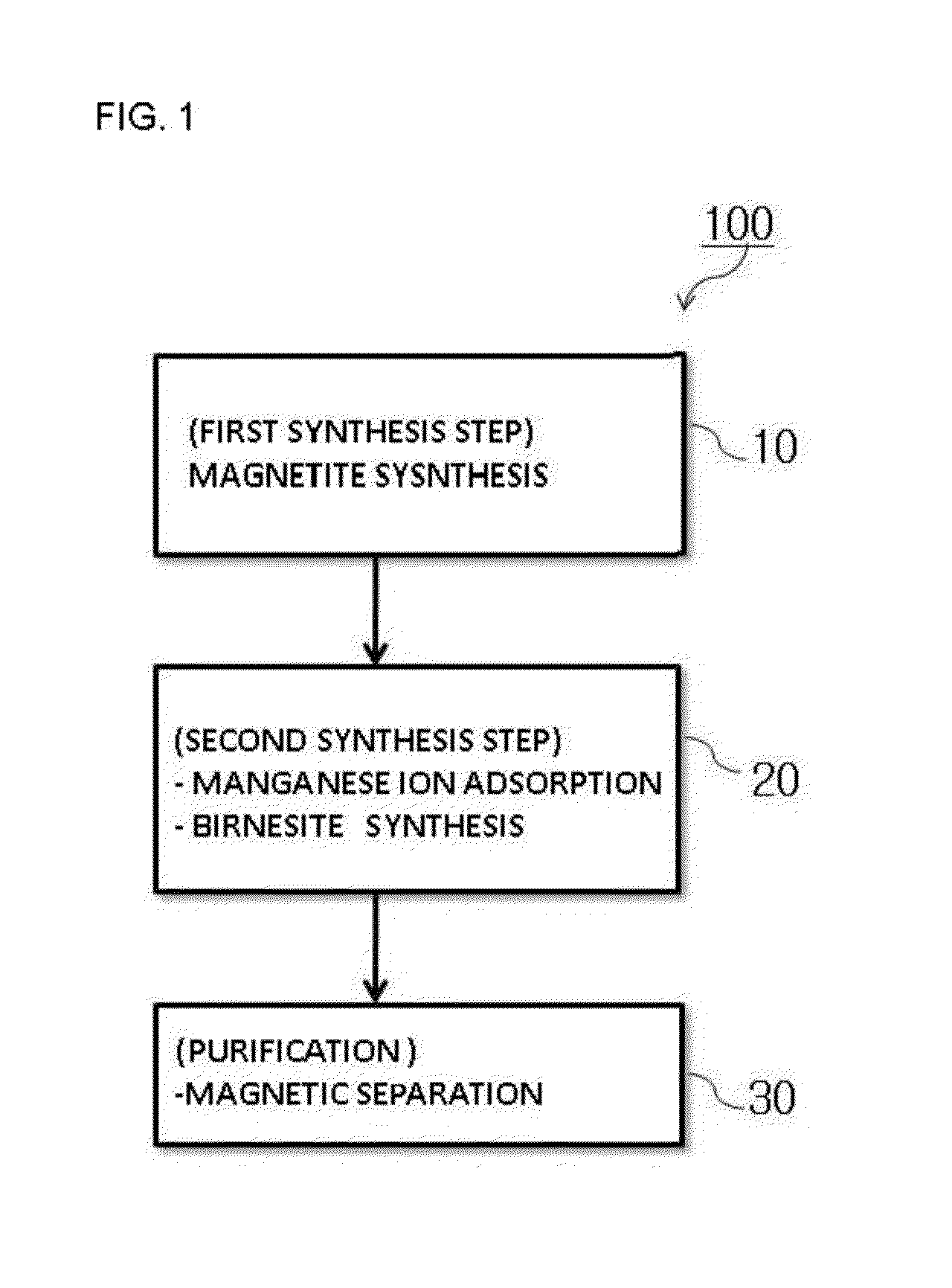
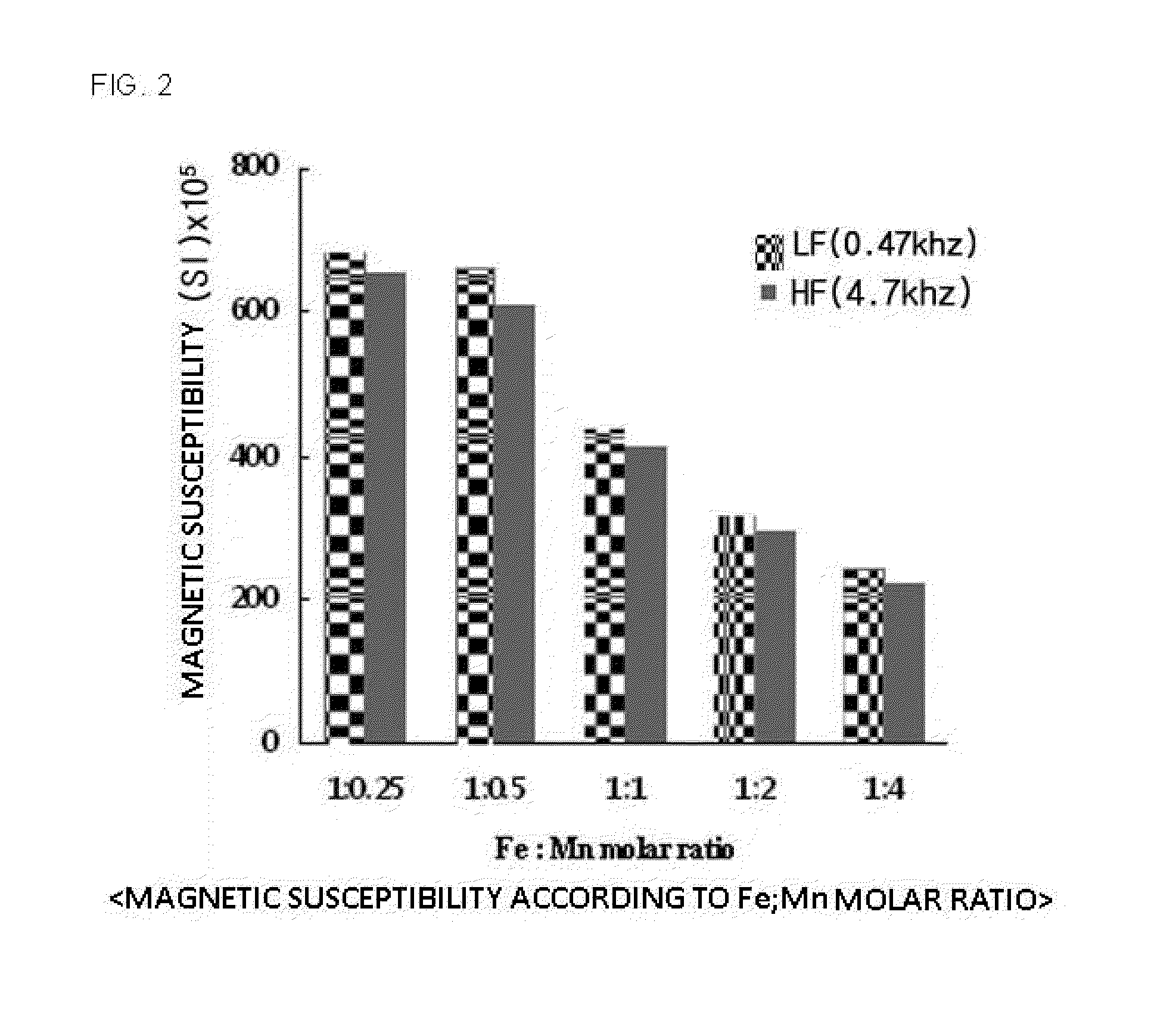
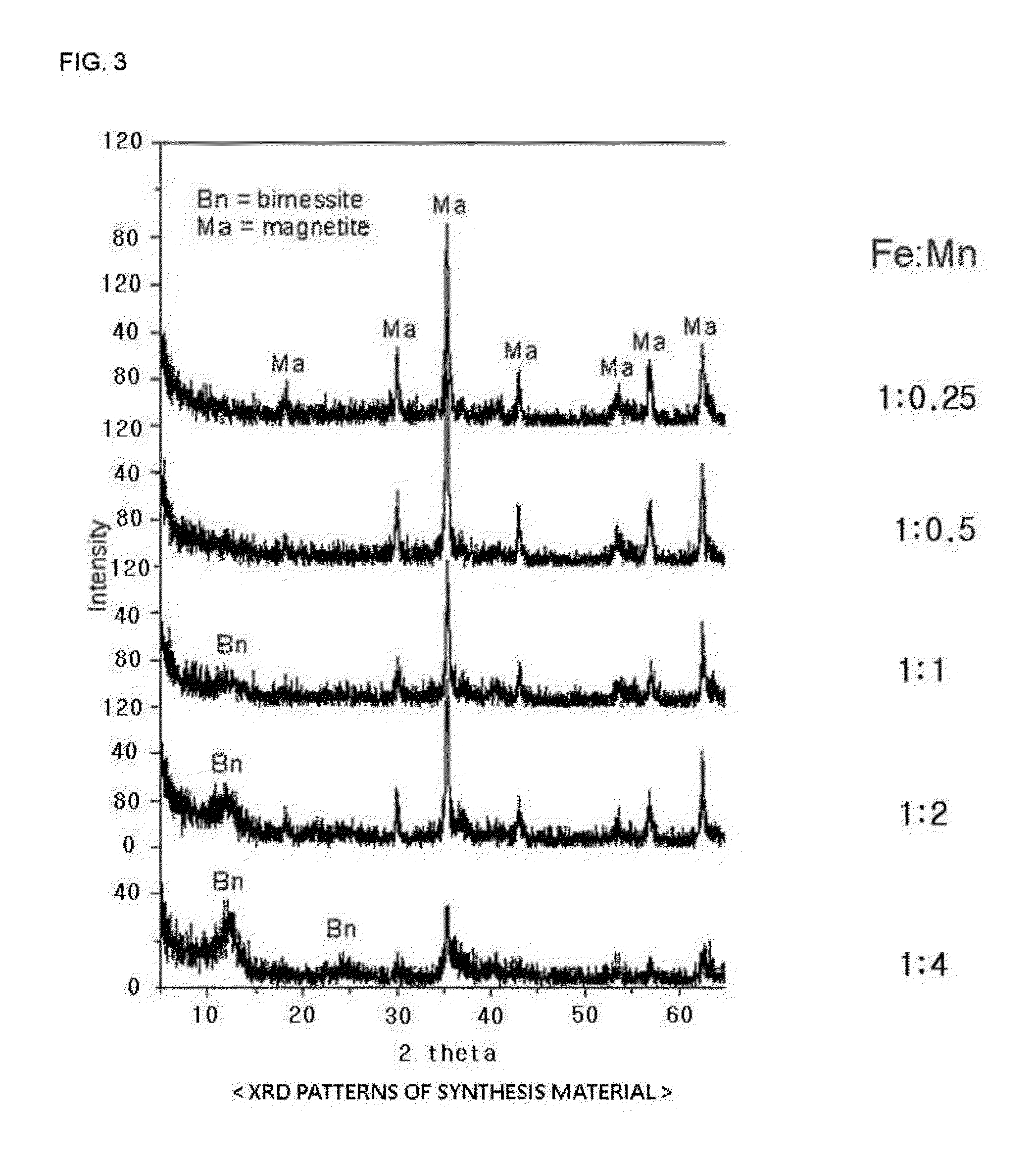
The present invention relates to a magnetite-birnessite mixture, to a synthesis method therefor, and to a water-treatment method using the same. The magnetite-birnessite mixture synthesis method according to the present invention includes: a first synthesis step in which magnetite is synthesized; a second synthesis step in which manganese is made to adsorb onto the surface of the magnetite by supplying manganese while maintaining a basic state in the presence of the magnetite, and then synthesizing birnessite on the surface of the magnetite by supplying an oxidizing agent and sodium, thereby synthesizing a mixture in which magnetite and birnessite are bound together; and a purification step in which the mixture of magnetite and birnessite is purified.
Owner:KOREA INST OF GEOSCI & MINERAL RESOURCES
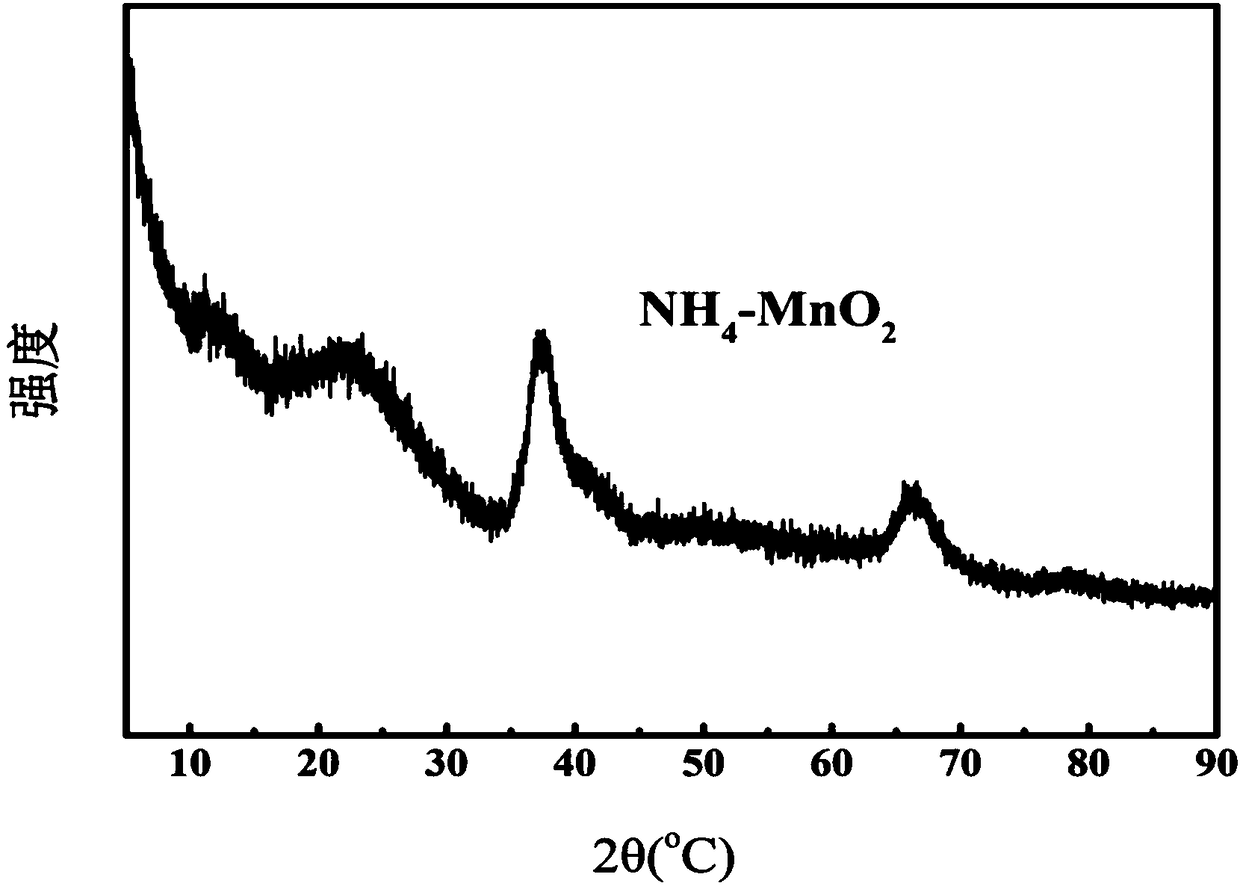
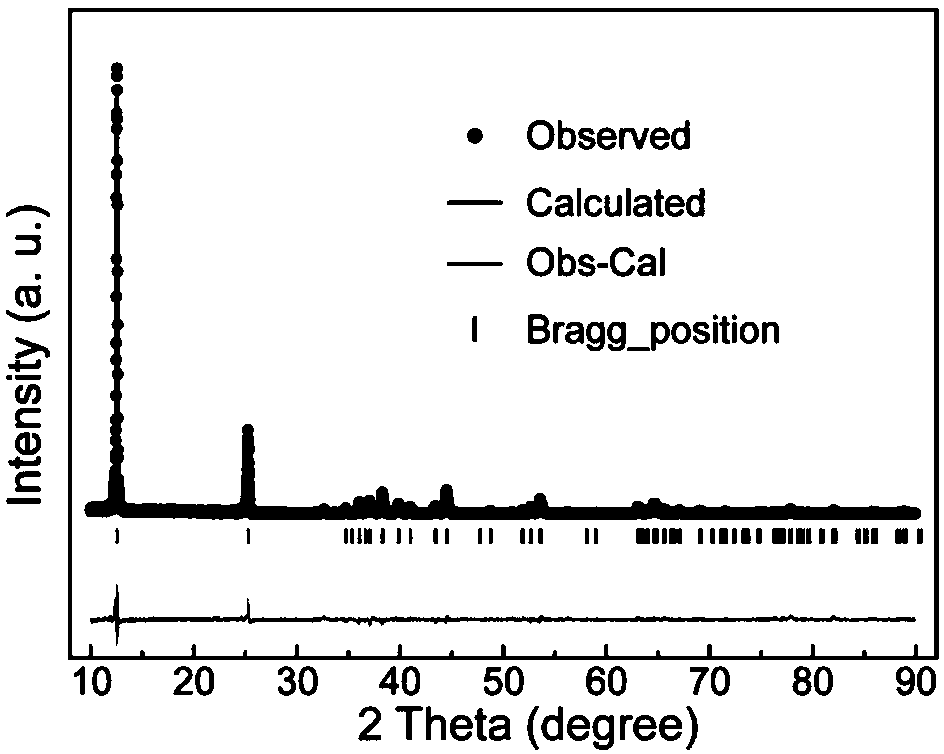
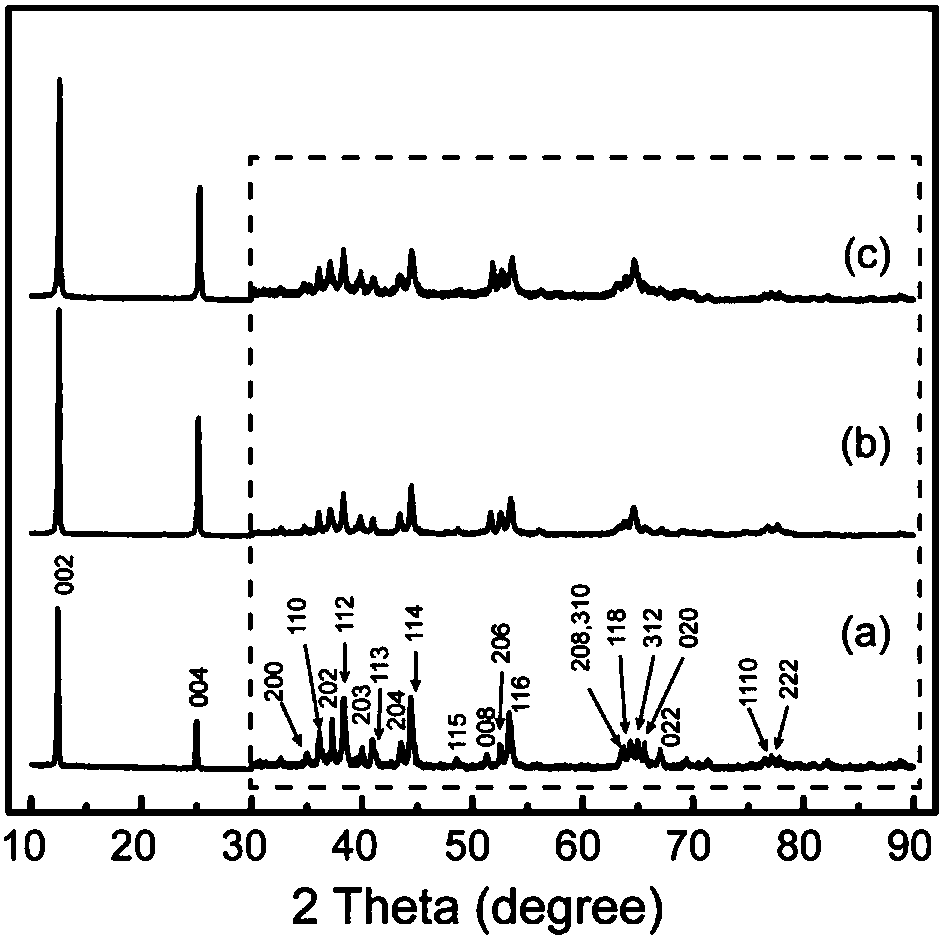
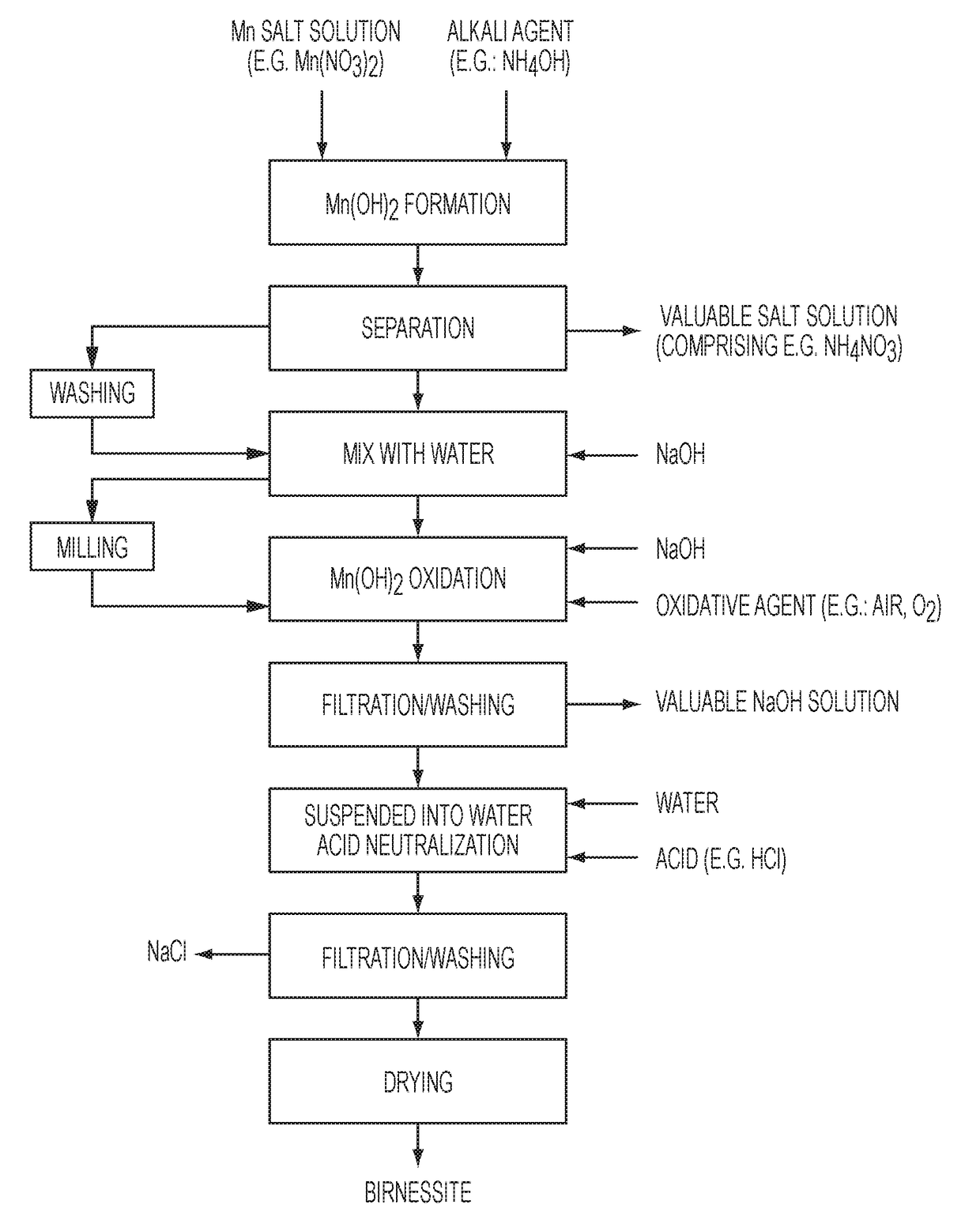
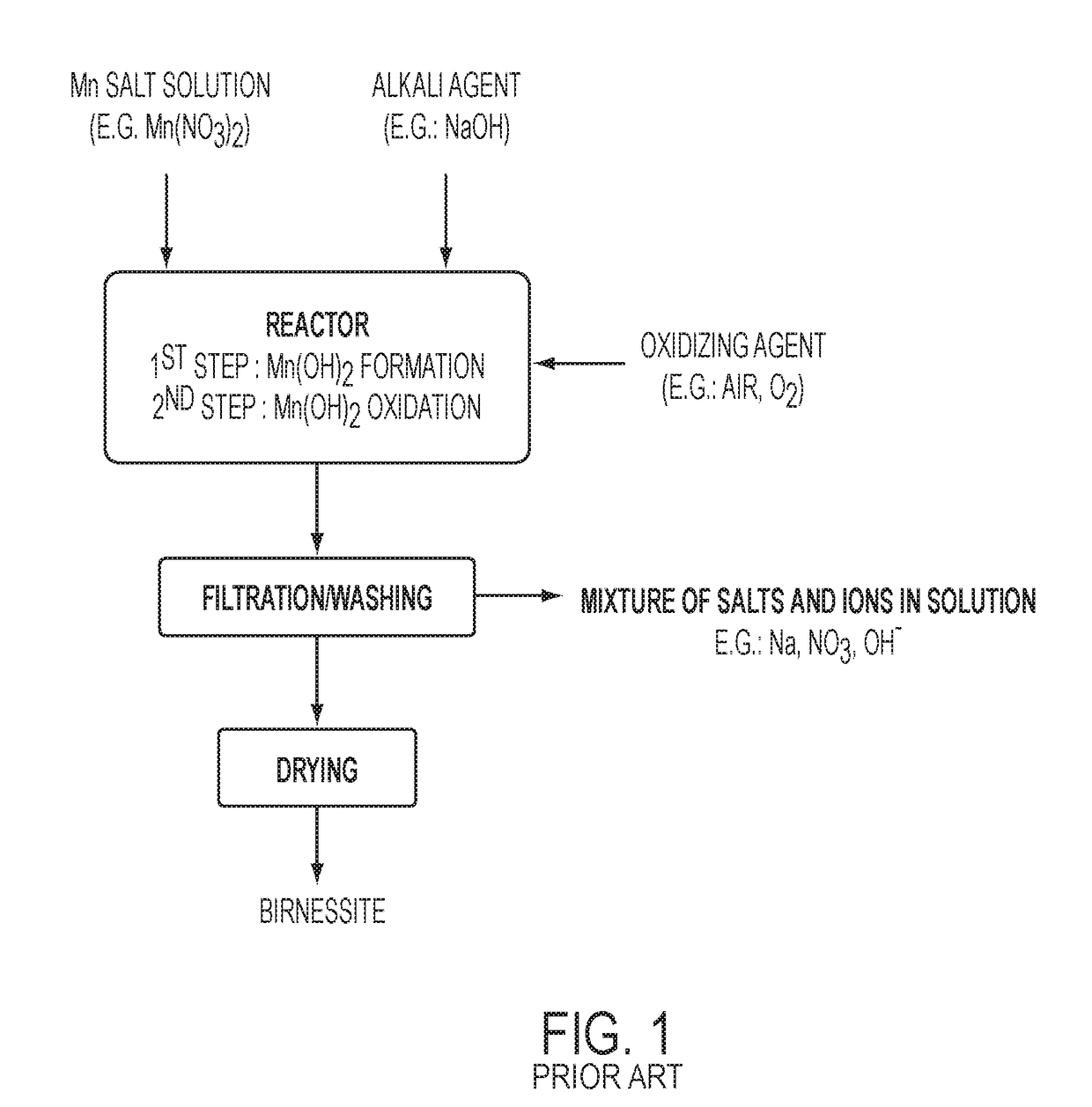
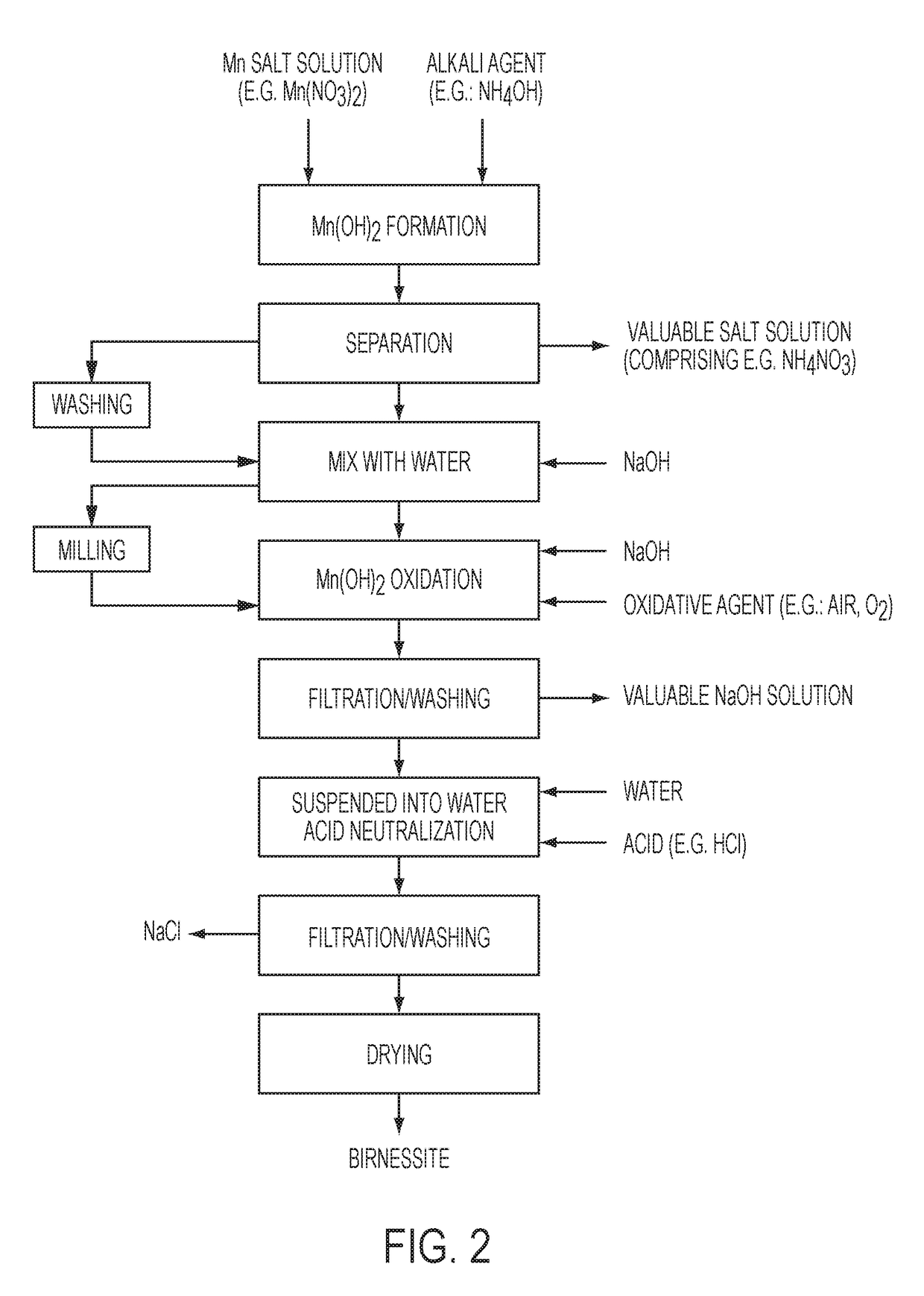
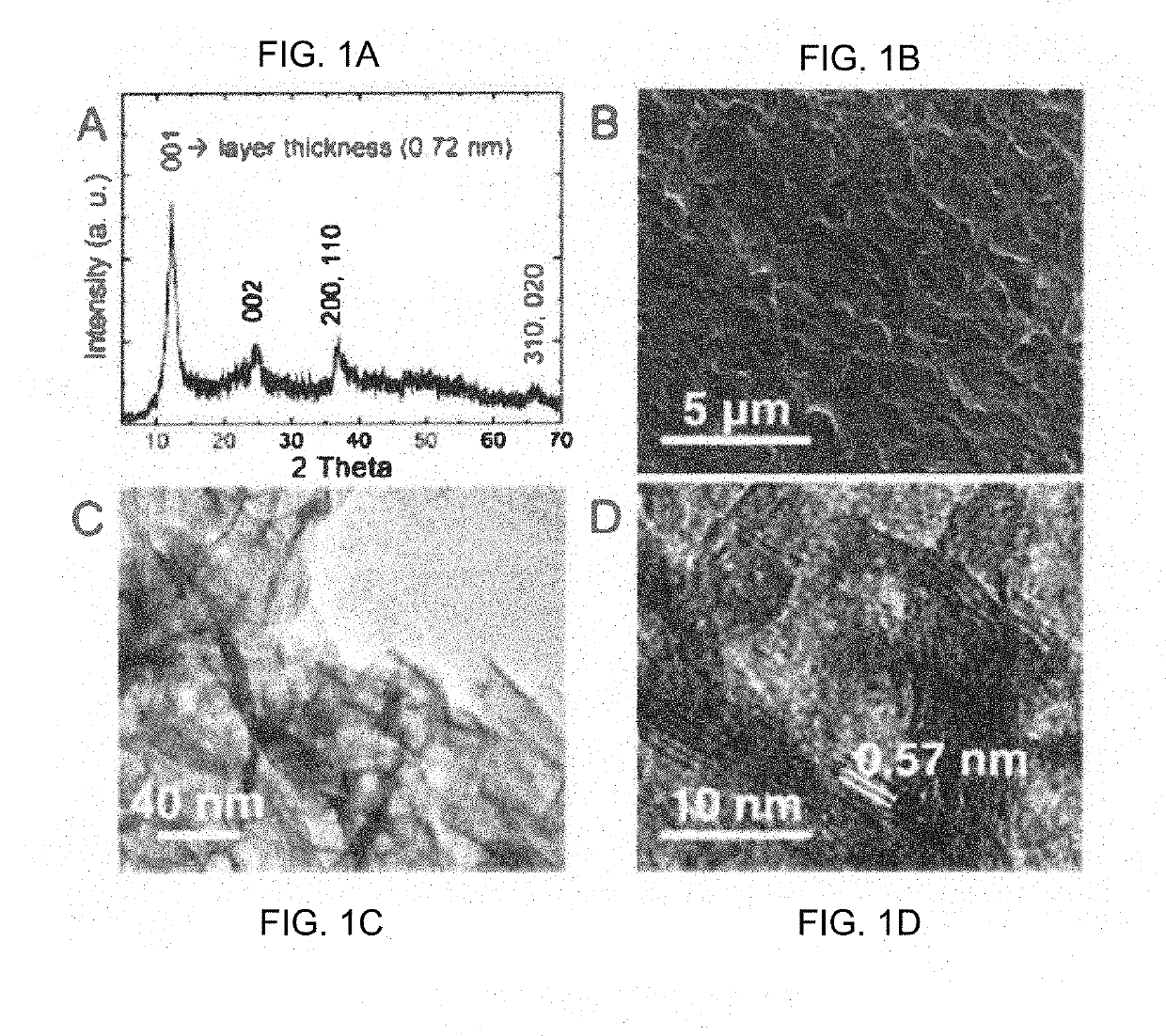
Highly Pure Birnessite and Method for the Production Thereof
A method of producing an oxide of manganese including reacting, in a first aqueous solution, a manganese salt and an alkali agent to form manganese hydroxide; separating the manganese hydroxide from the first solution; mixing the manganese hydroxide into an aqueous medium to form a manganese hydroxide suspension; mixing the manganese hydroxide suspension with alkali metal hydroxide to form a second aqueous solution; and oxidizing the manganese hydroxide in the second aqueous solution to form an oxide of manganese. The dried oxide of manganese includes birnessite, a maximum of 20% hausmannite, and a maximum of 10% feitknechtite, may further include a maximum of 400 ppm of anions, may have a specific surface area of at least 25 m2 / g, and may have an average oxidation state of greater than 3.5.
Owner:PRINCE ERACHEM SPRL
Low-odor polyurethane sealant for automobiles and preparation method of low-odor polyurethane sealant
InactiveCN109280527AGood effectDecompose fullyNon-macromolecular adhesive additivesPolyureas/polyurethane adhesivesBirnessitePlasticizer
The invention provides a low-odor polyurethane sealant for automobiles and a preparation method of the low-odor polyurethane sealant. The preparation method comprises the following steps: preparing alayered mesoporous birnessite-type MnO2 nanosphere supported deodorant filler, and carrying out vacuum mixing and stirring on the layered mesoporous birnessite-type MnO2 nanosphere supported deodorantfiller with a isocyanate terminated polyurethane prepolymer in a high-speed stirring kettle, then adding a thixotropic agent, a plasticizer and a curing accelerator, and carrying out vacuum stirringto obtain the mesoporous birnessite-type MnO2 modified low-odor polyurethane sealant. The method provided by the invention overcomes the technical problem that heavy odor is generated when a polyurethane sealant prepared in the prior art is used for automotive interior decorations. A deodorant is supported in layered mesoporous birnessite-type MnO2 nanospheres, so that small molecule volatile matter can be fully adsorbed and decomposed, birnessite-type MnO2 can convert formaldehyde in the automobiles into carbon dioxide and water at room temperature, and odor diffusion of the polyurethane sealant can be remarkably reduced.
Owner:CHENDU NEW KELI CHEM SCI CO LTD
Mixed Material Cathode for Secondary Alkaline Batteries
A secondary alkaline battery using manganese dioxide is described. The battery includes a mixed cathode material with birnessite-phase manganese dioxide or electrolytic manganese dioxide (EMD), a bismuth compound and a copper compound selected from the group consisting of elemental copper and a copper salt. In some embodiments, a conductive carbon and / or a binder may also be included.
Owner:RES FOUND THE CITY UNIV OF NEW YORK
Preparation method of brain-coral-shaped birnessite type manganese dioxide
InactiveCN102120619BImprove structural stabilityShape unchangedNanotechnologyManganese oxides/hydroxidesStructural stabilityPotassium permanganate
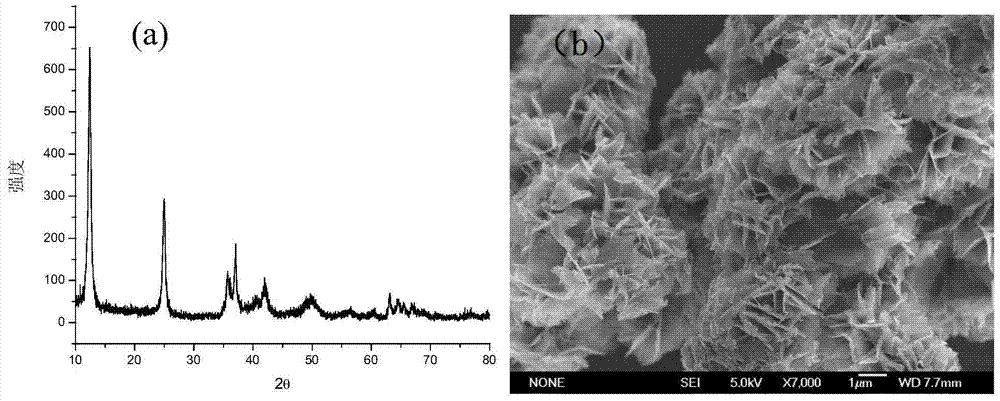
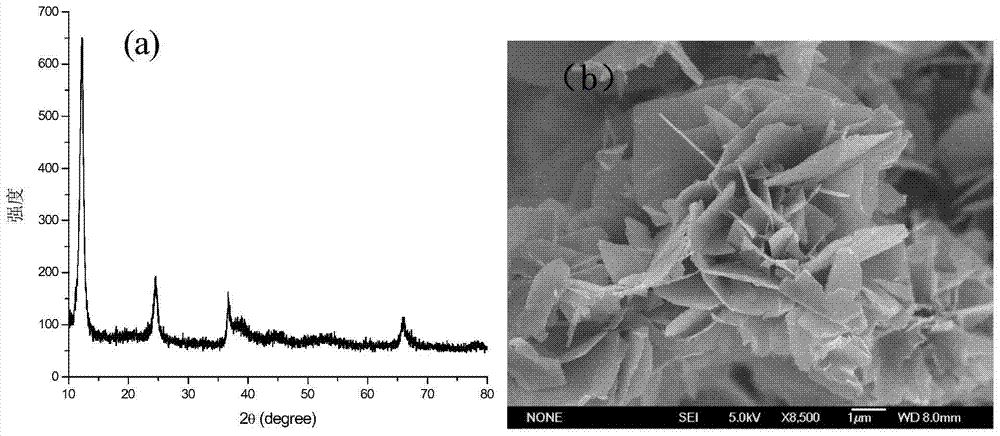
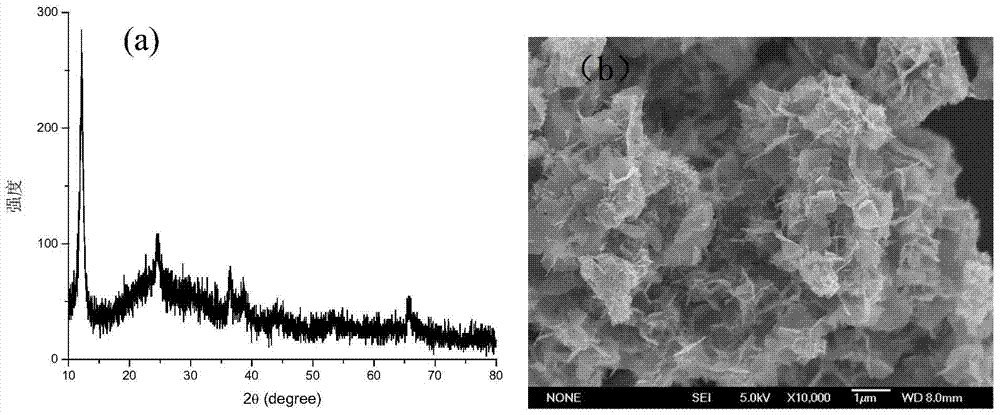
The invention discloses a preparation method of brain-coral-shaped birnessite type manganese dioxide, comprising the steps of: subjecting potassium permanganate and manganese sulfate, which are cheap and serve as reactants, to redox reaction in a water solution at room temperature, and obtaining the brain-coral-shaped birnessite type manganese dioxide by controlling the pH values of a manganese sulfate solution and a potassium permanganate solution to be 0-2 before mixture and controlling the pH of a reaction system in the mixing process of the two reactant solutions to be changeless. The preparation method provided by the invention is free from organic solvents, surfactants and templates as well as hydrothermal treatment, and has the advantages of simple preparation process, low cost, mild reaction conditions and environmental friendliness. The brain-coral-shaped birnessite type manganese dioxide prepared in the invention has the specific surface area reaching 140-220m<2> / g, the porevolume of 0.1-1cm<3> / g, the pore diameter of 15-50nm, and favorable structural stability.
Owner:HEBEI NORMAL UNIV
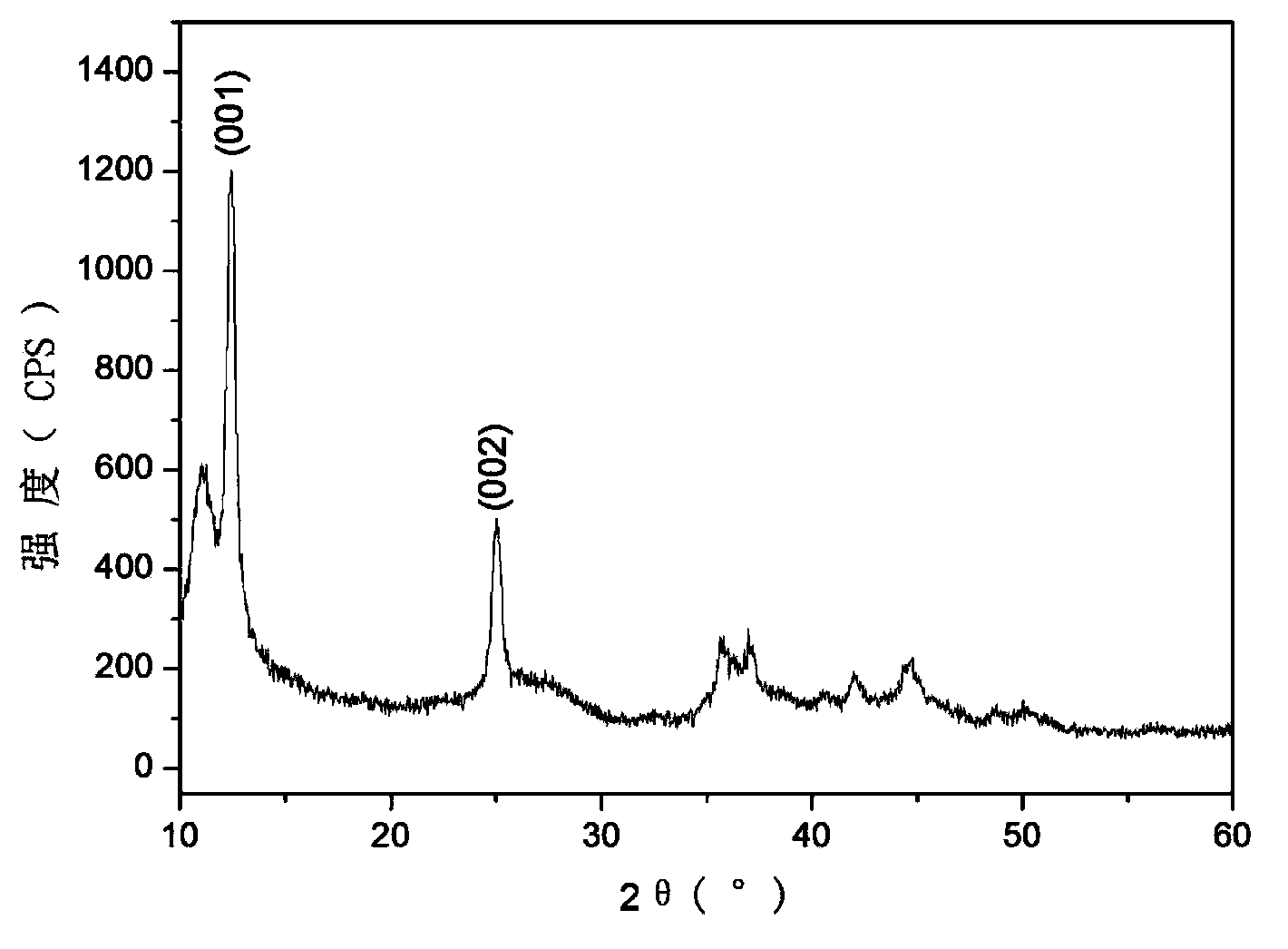
Photochemically-assisted synthesis of layered birnessite (MNO2) nanosheets
ActiveUS20190284061A1Easy to stackIncreased formationMaterial nanotechnologyPositive electrodesBirnessitePhotochemistry
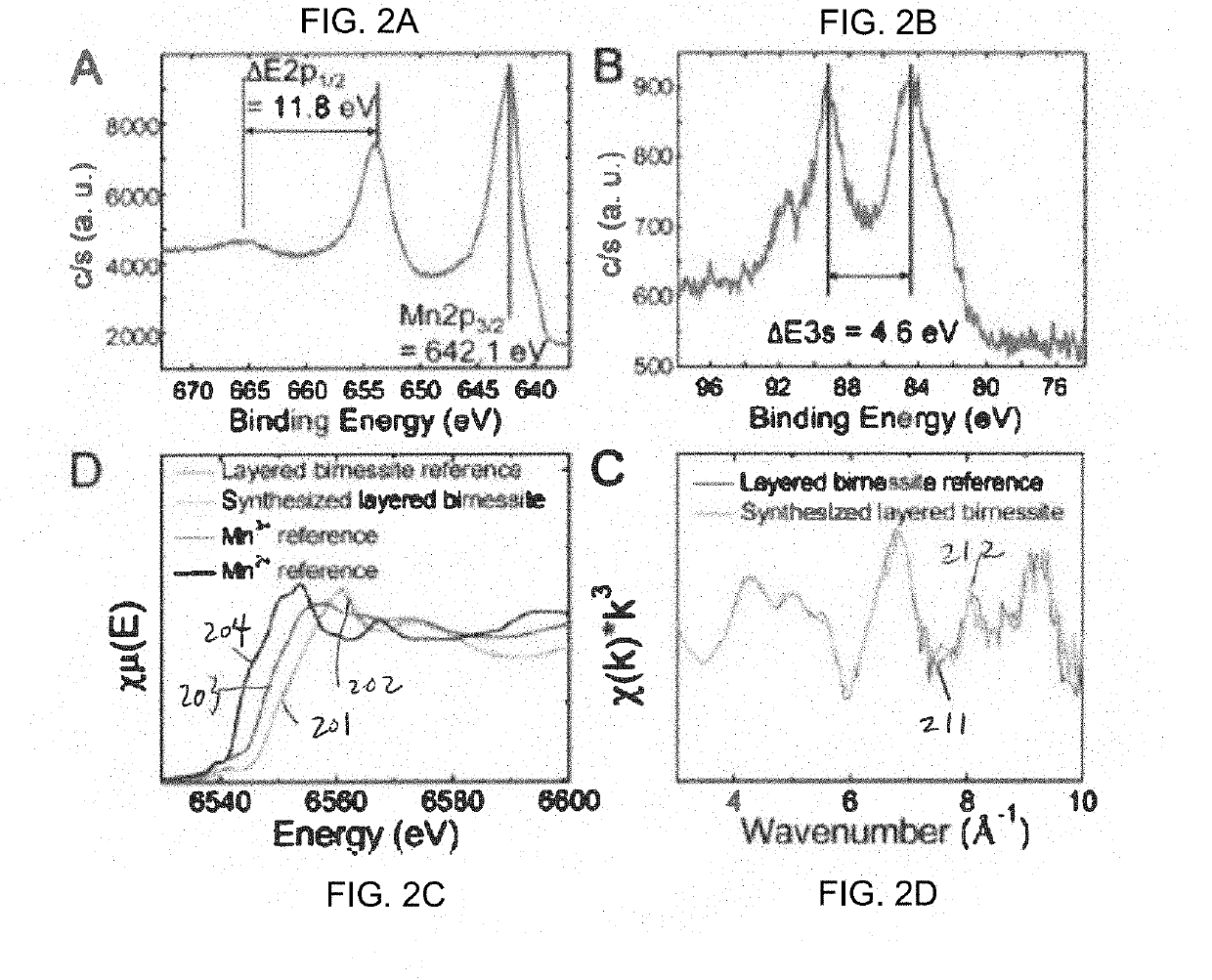
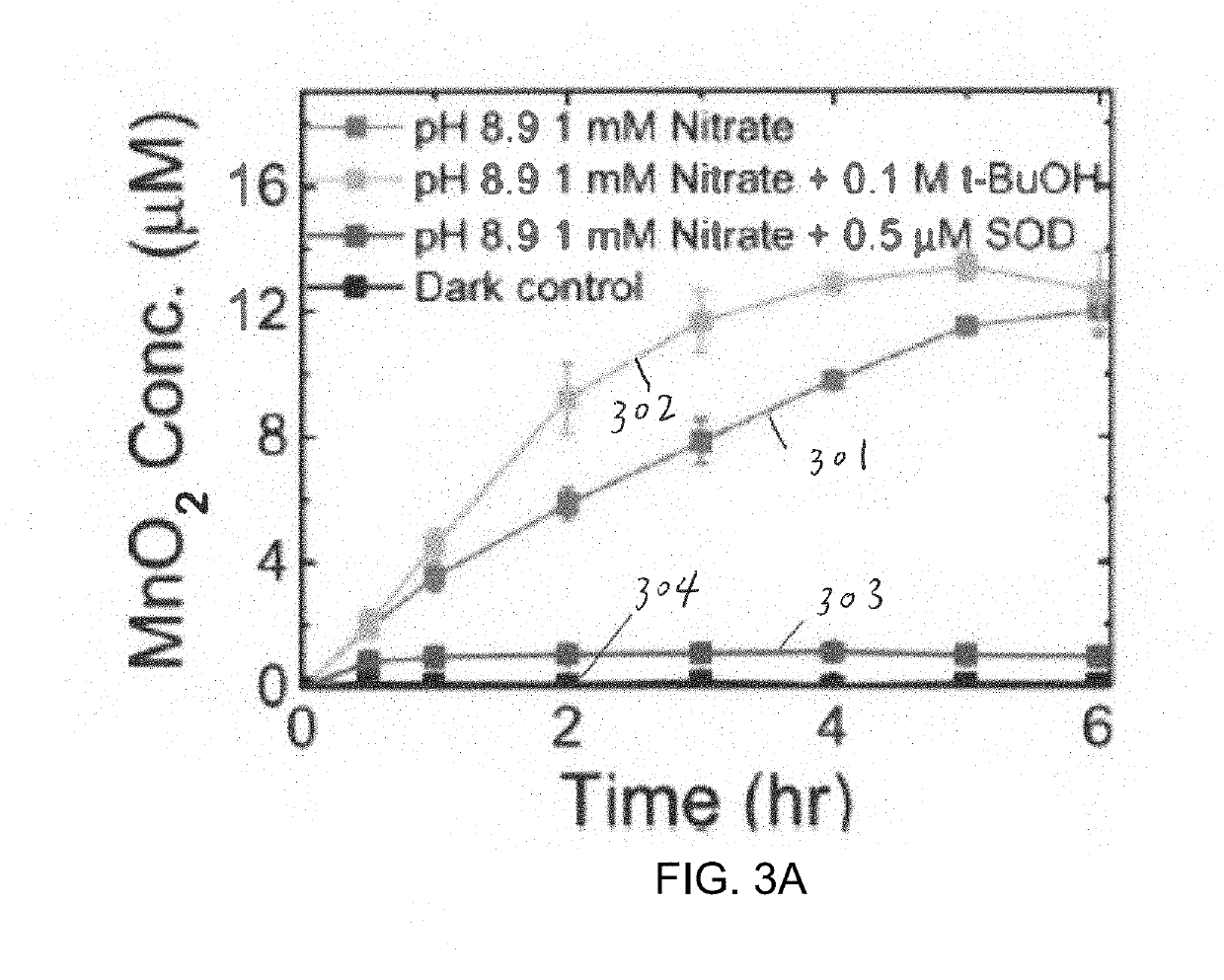
A method of forming birnessite δ-MnO2 nanosheets is provided. The method includes oxidizing manganese (Mn2+) in the presence of a source of nitrate and a light source.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Manganite/metalloporphyrin compound layered sandwich nanometer material suitable for electrocatalysis water oxidation
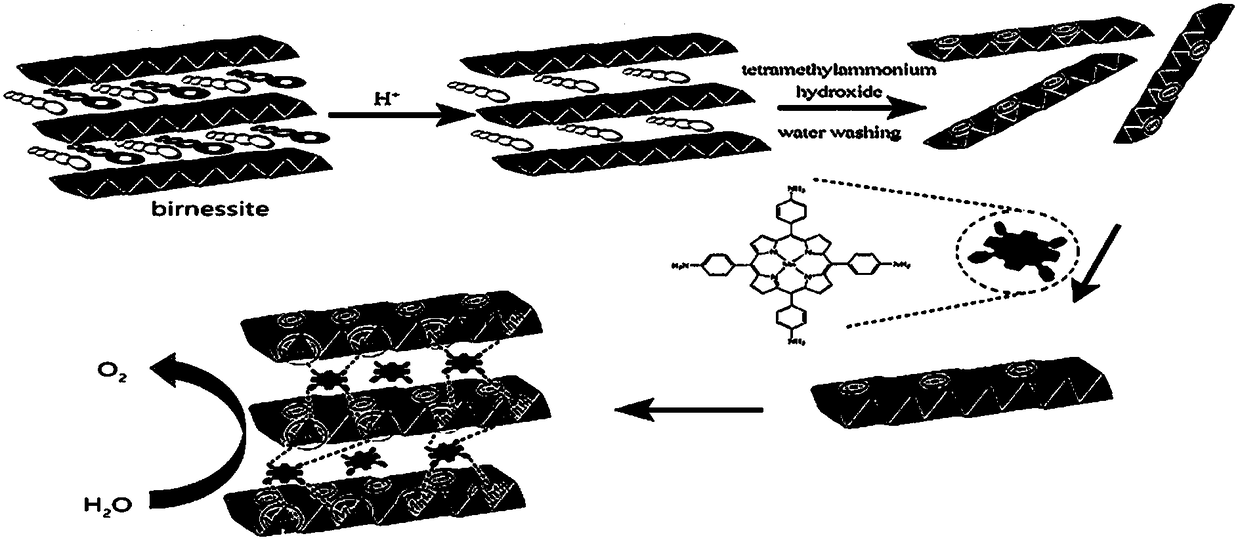
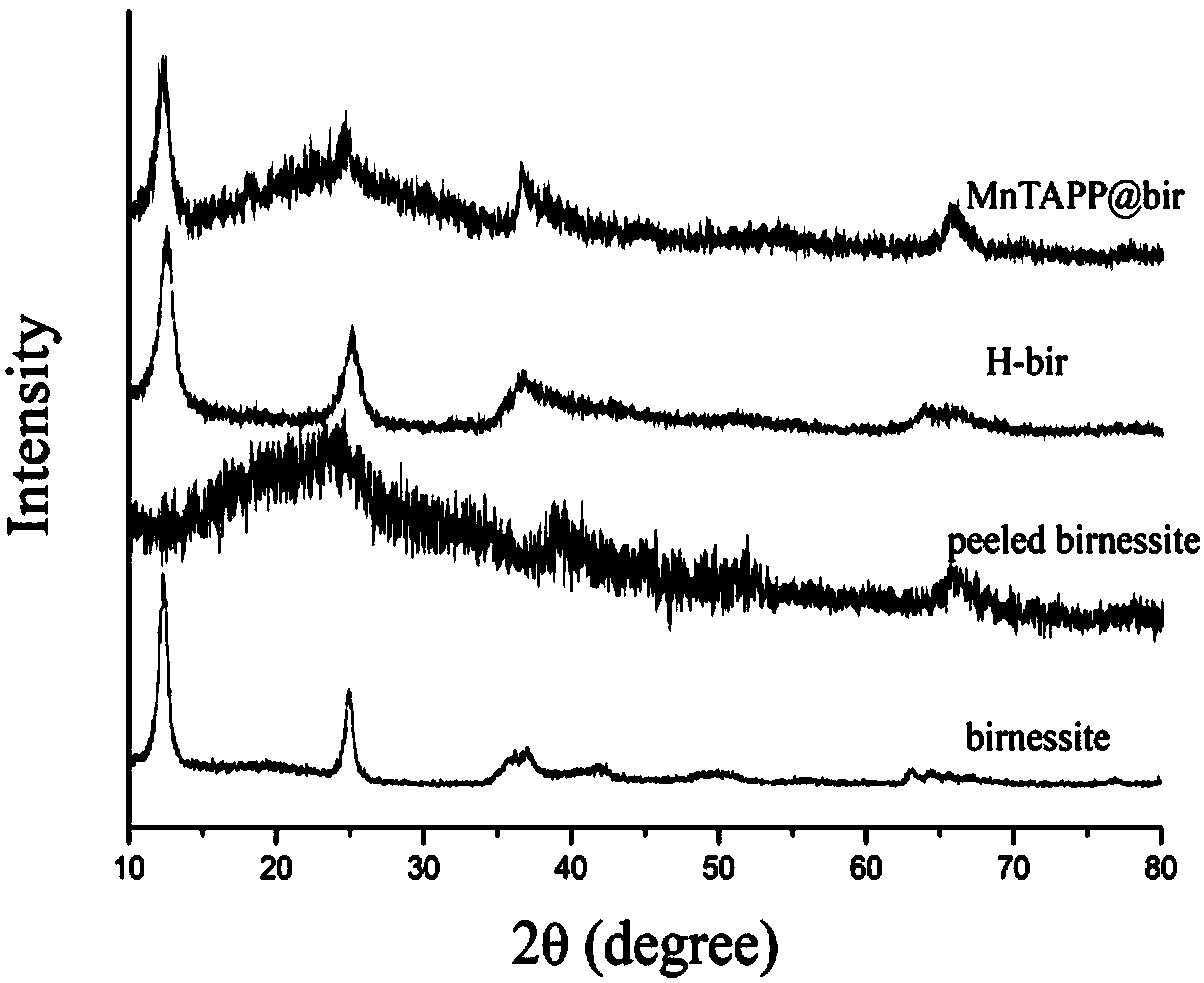
InactiveCN109126889AImprove structural stabilityImprove conductivityOrganic-compounds/hydrides/coordination-complexes catalystsElectrodesDecompositionPorphyrin
The invention discloses a preparing method of a manganite / metalloporphyrin compound layered sandwich nanometer material suitable for electrocatalysis water oxidation, and belongs to the field of photoelectrocatalysis decomposition water hydrogen production catalysis materials. The preparing method includes the steps of synthesizing high-purity birnessite type delta-MnO2 through a complexing agent,and stripping into a single-layer birnessite type delta-MnO2 sheet; synthesizing amine porphyrin with electropositivity and placing the sheet and the amine porphyrin in a flask to be mixed and recombined into the novel layered sandwich structure nanometer conductive compound catalysis material. The obtained material presents excellent electrocatalysis water oxidization (OER) performance, the electric potential is kept at 0.91 V when the electric current density is 5 mA cm<-2> and is much increased compared with birnessite type delta-MnO2, the duration reaches 4000 s under the condition of thelarge electric current density of 10 mA cm<-2>, and quite high stability can be kept.
Owner:HUNAN UNIV
Manganese dioxide and curable composition containing same
InactiveCN104284863AReduce swellingReduce intensityOther chemical processesIron compoundsBirnessiteWarm water
Provided are: manganese dioxide which can cure a liquid polysulfide polymer and enables the production of a cured article that is rarely swollen or rarely undergoes the deterioration in strength even when immersed in warm water for a long period; and a curable composition containing the manganese dioxide. The manganese dioxide can be used as a curing agent for a liquid polysulfide polymer, and is characterized by comprising monoclinic cation-substituted birnessite-type manganese dioxide produced by substituting each of sodium ions existing between layers of monoclinic sodium birnessite represented by the formula: Na0.55Mn2O4·1.5H2O by a monovalent to trivalent cation.
Owner:TOKAN MATERIAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com