Manganese dioxide and curable composition containing same
A manganese dioxide and composition technology, which is applied in the field of hardeners for liquid polysulfide polymers, can solve the problems of reduced performance of hardened products, and achieve the effects of reducing swelling and strength.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0082] The present invention will be explained in detail with reference to the following examples. Unless otherwise stated, "parts" given hereinafter are "parts by weight" of the weight standard.
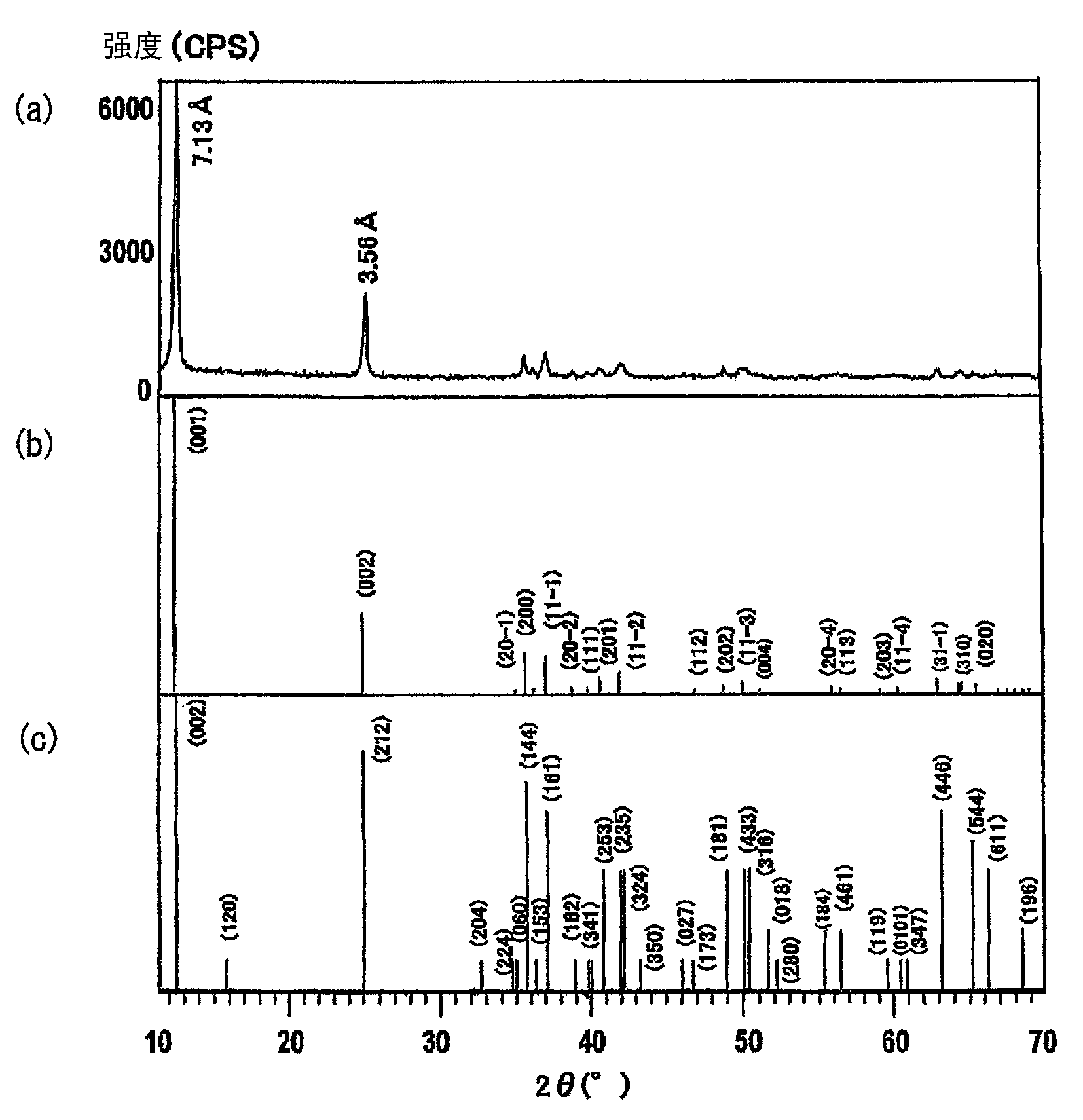
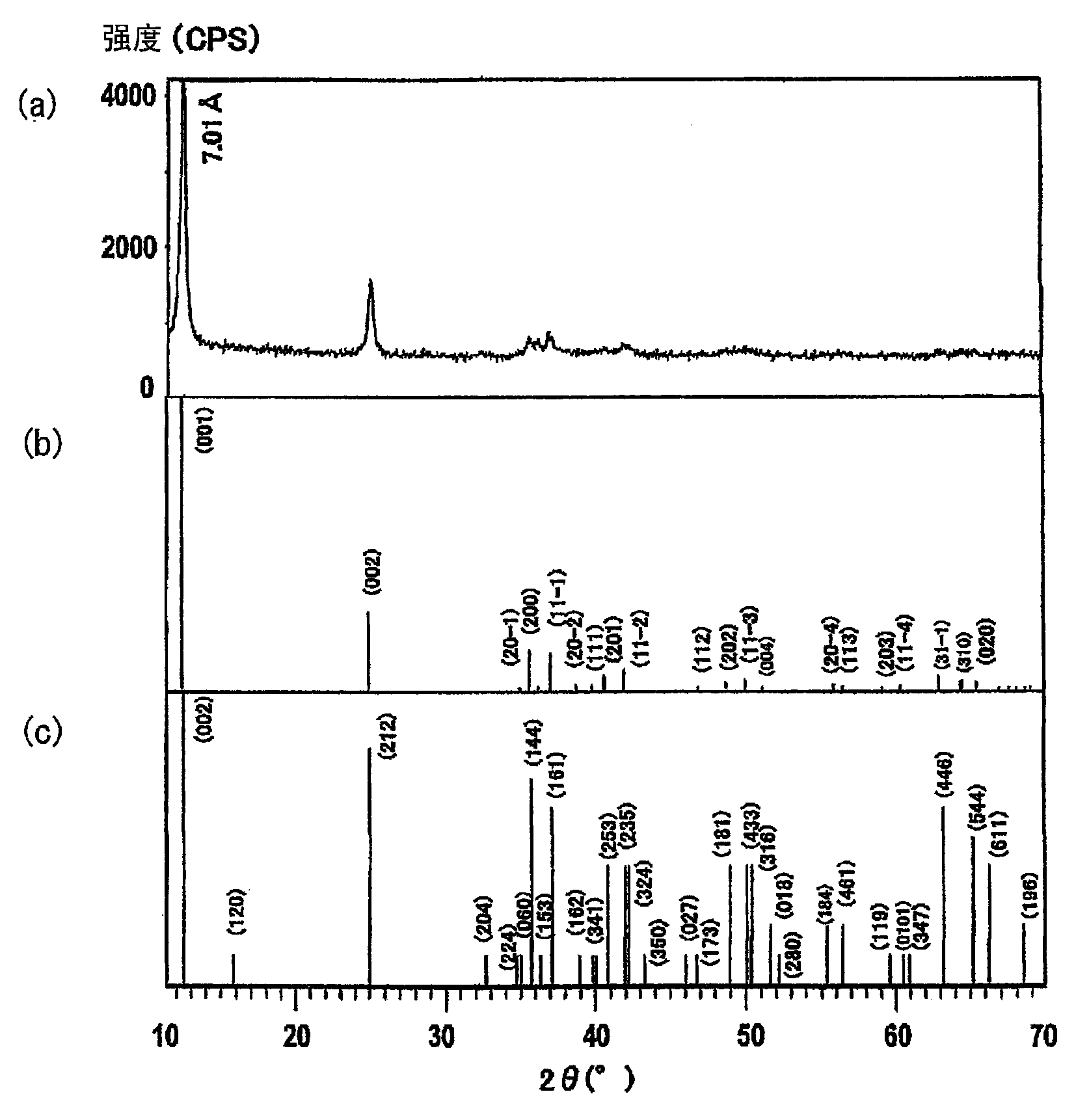
[0083] In order to identify the crystal structure and calculate the interlayer distance of manganese dioxide, powder X-ray diffraction (X'Pert PRO MRD produced by Spectris Co., Ltd.) was performed, thereby obtaining an X-ray diffraction chart. The crystal structure was identified by comparison with a standard diffraction peak chart, and the interlayer spacing was calculated based on the assumption that the interplanar spacing of the peak reflected at index 001 corresponds to that of the birnessite structure.
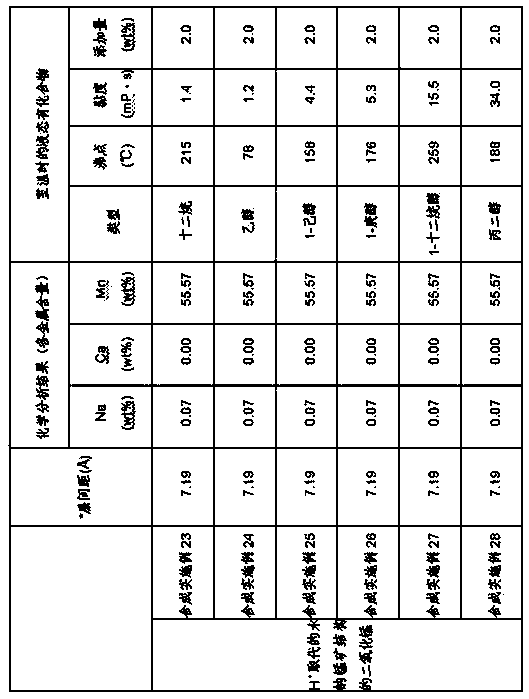
[0084] To calculate the chemical composition ratio, quantitative determination was performed by the Na atomic absorption method, the oxidation-reduction titration method for Mn, and the ICP emission spectrochemical analysis (ICP-AES) method for other metals. The chemical anal...
Synthetic example 2
[0091]400 parts of the filtered product (1) similar to that obtained in Synthesis Example 1 was added to a previously prepared aqueous solution of 441 parts of calcium chloride dihydrate and 3000 parts of distilled water, and stirred for 24 hours. Next, the obtained suspension was filtered and washed with water until the conductivity of the filtrate became 100 microSiemens (μS / cm). Then, the filtered product was collected, dried at 80° C. for 10.5 hours, and then ground in a mortar to obtain a sample of Synthesis Example 2.
[0092] figure 2 The X-ray diffraction pattern of the sample obtained in Synthesis Example 2 is shown in (a). For comparison, figure 2 (b) shows the standard monoclinic sodium birnessite Na 0.55 mn 2 o 4 ?1.5H 2 X-ray diffraction pattern of O, and figure 2 (c) shows orthorhombic sodium birnessite Na 4 mn 14 o 27 ?9H 2 Standard X-ray diffraction pattern of O. figure 2 (a) Analysis with figure 1 (a) Similar. Analysis showed that the sample ...
Synthetic example 14
[0116] 400 parts of the filtered product (1) similar to that obtained in Synthesis Example 1 was added to a previously prepared aqueous solution consisting of 799.9 parts of strontium chloride hexahydrate and 3000 parts of distilled water, and stirred for 24 hours. Next, the obtained suspension was filtered and washed with water until the conductivity of the filtrate became 100 microSiemens (μS / cm). The filtered product was then collected, dried at 80° C. for 10.5 hours, and then ground in a mortar to obtain a sample. This sample was evaluated similarly to Synthesis Example 1. The interlayer spacing and chemical composition ratio are shown in Table 1. From these findings, Synthesis Example 14 is monoclinic birnessite containing 0.01% by weight of sodium with interlayer-introduced strontium ions.
[0117] [Synthesis Example 15]
[0118] 400 parts of the filtered product (1) similar to that obtained in Synthesis Example 1 was added to a previously prepared aqueous solution c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com