Chargeable and dischargeable aqueous aluminum ion battery and preparation process thereof
An aluminum ion battery, charging and discharging technology, applied in the direction of battery electrodes, secondary batteries, circuits, etc., can solve the problems of complex structure and impracticality, and achieve good conductivity, excellent chemical stability, and low pollution effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
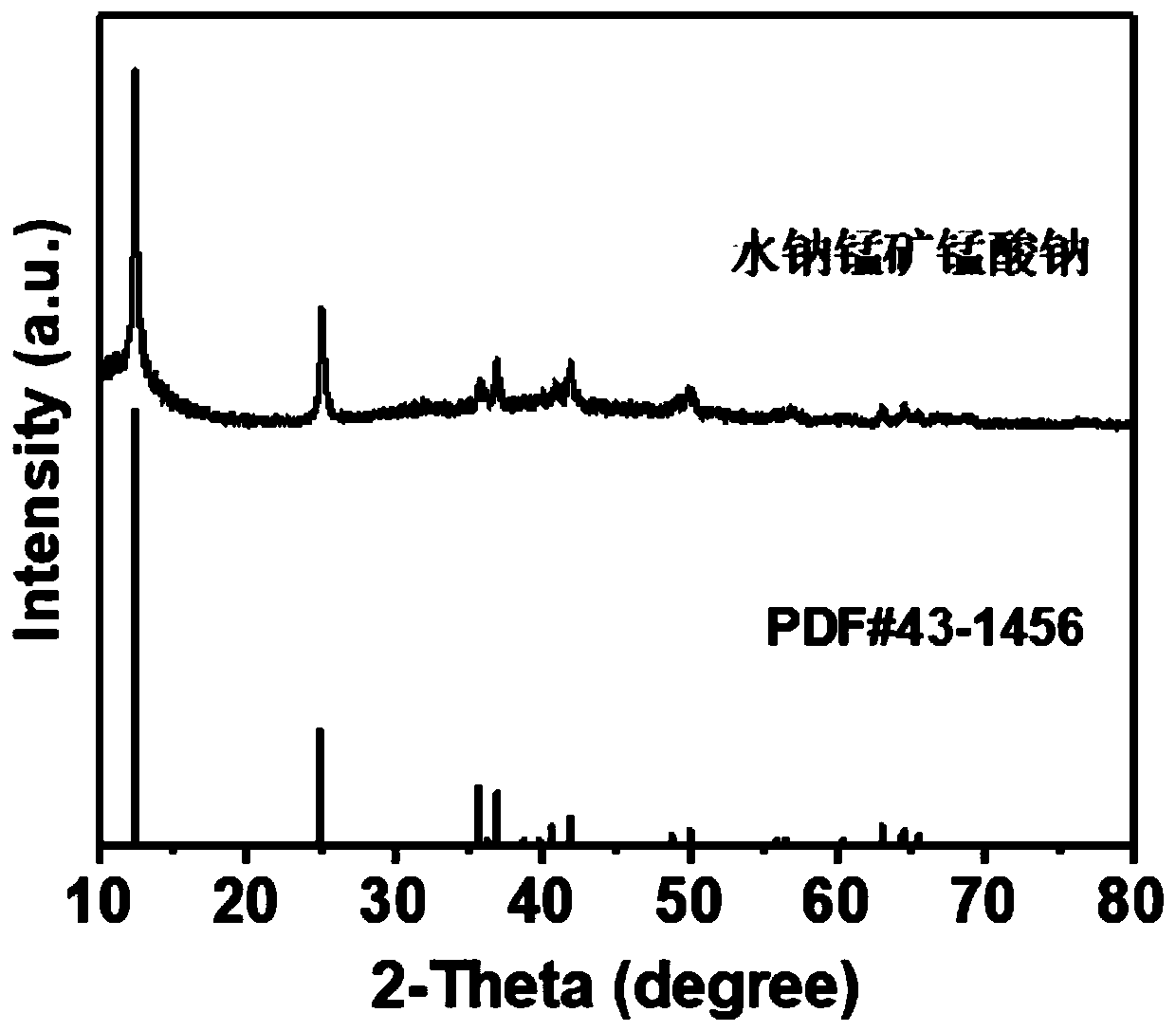
[0023] Preparation of battery positive electrode: 0.3M Mn(NO 3 ) 2 The solution was added to 0.6M NaOH and 2M H 2 o 2 solution, after stirring for 0.5h, add 2M NaOH, put the mixture into the reaction kettle, and react at 150°C for 16h to obtain the Na of birnessite 0.55 mn 2 o 4 1.5H 2 O. Mix this birnessite sodium manganate material with Super P conductive agent and polyvinylidene fluoride (PVDF) binder in N-methylpyrrolidone at a mass ratio of 70:20:10 to make a slurry, and coat it on stainless steel On the sheet, dry in a vacuum oven for 10h to make an electrode sheet.
[0024] Electrolyte preparation: Weigh 0.95 g of aluminum trifluoromethanesulfonate powder in a glove box and place it in a reagent bottle, add 1 mL of deionized water, stir for 12 hours, and prepare a 2M aluminum trifluoromethanesulfonate electrolyte.
[0025] Preparation of aluminum negative electrode: mix aluminum chloride and ionic liquid according to the preferred molar ratio of 1.3:1, and let s...
Embodiment 2
[0028] Others are as in Example 1.
[0029] Metal aluminum is directly used as the negative electrode of the battery without immersion in ionic liquid.
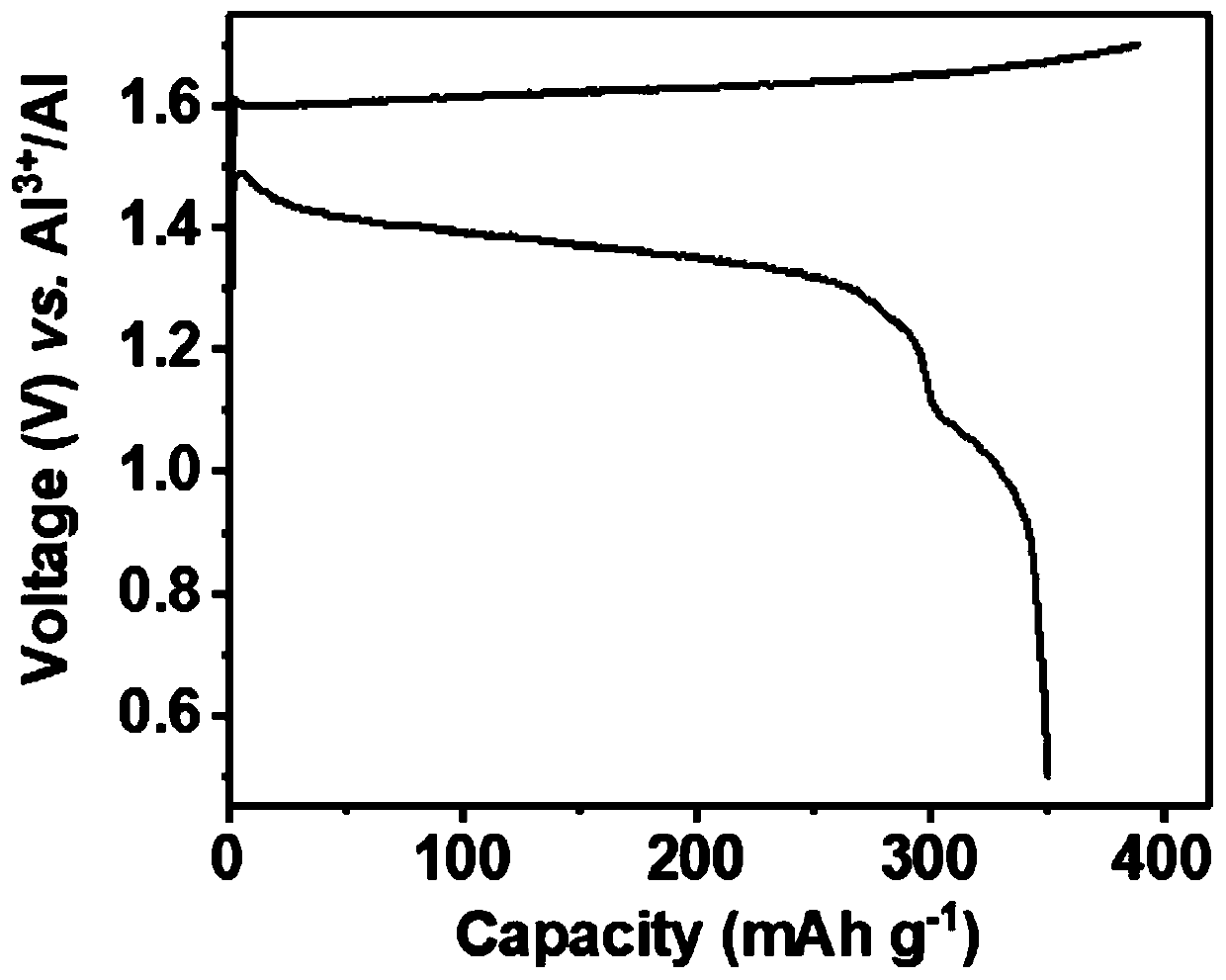
[0030] Battery at 50mAg -1 Charge and discharge at constant current, the charge and discharge voltage range is 0.5–1.8V, and the discharge specific capacity is 365mAh g -1 , The average discharge voltage is 1.08V, and it can be charged and discharged more than 3 times.
Embodiment 3
[0032]Others are as in Example 1.
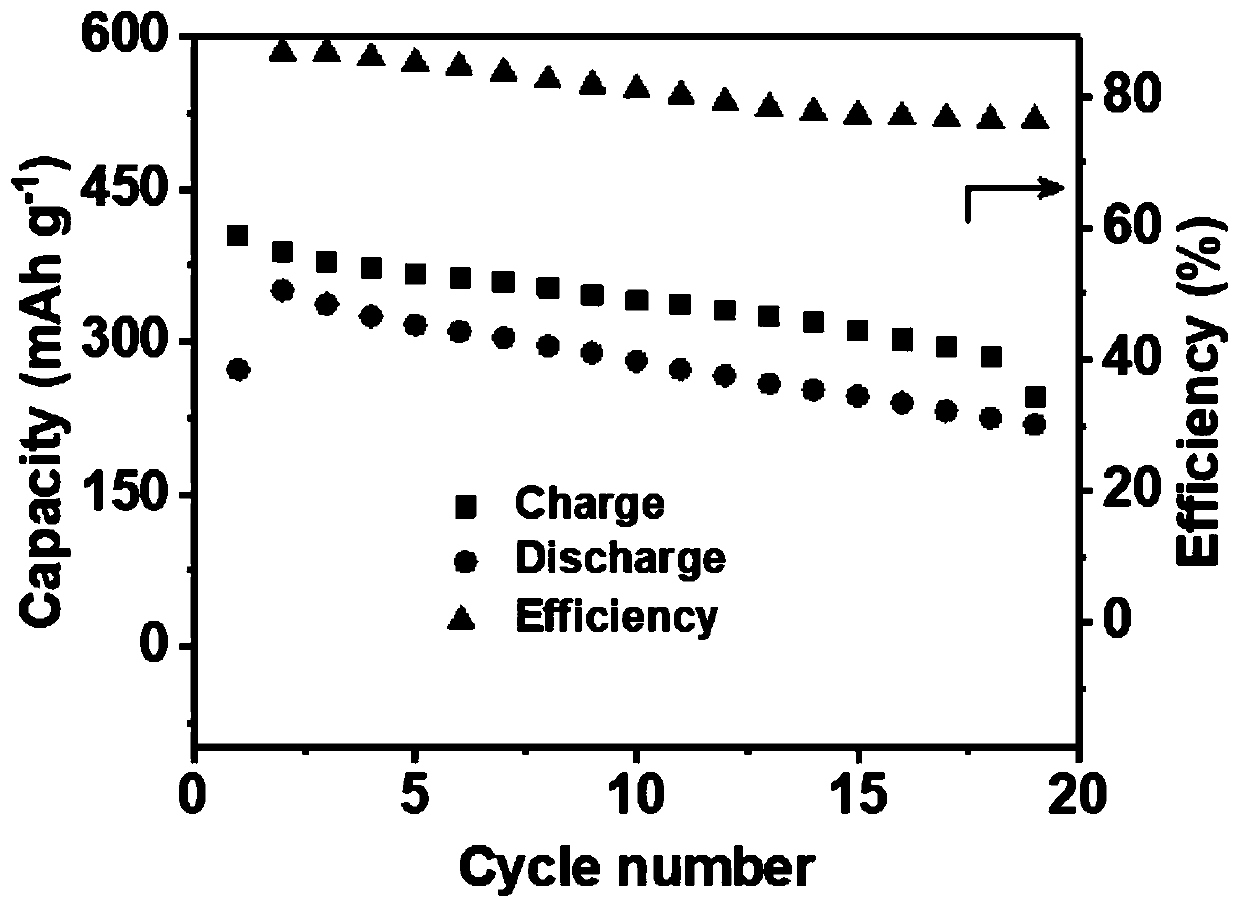
[0033] With 5M aluminum trifluoromethanesulfonate aqueous solution as the electrolyte, the battery operates at 50mA g -1 Charge and discharge at a constant current, the charge and discharge voltage range is 0.5–1.7V, and the discharge specific capacity is 137mAh g -1 , the average discharge voltage is 1.38V, and the remaining specific capacity is 64mAh g after 10 charge-discharge cycles -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com