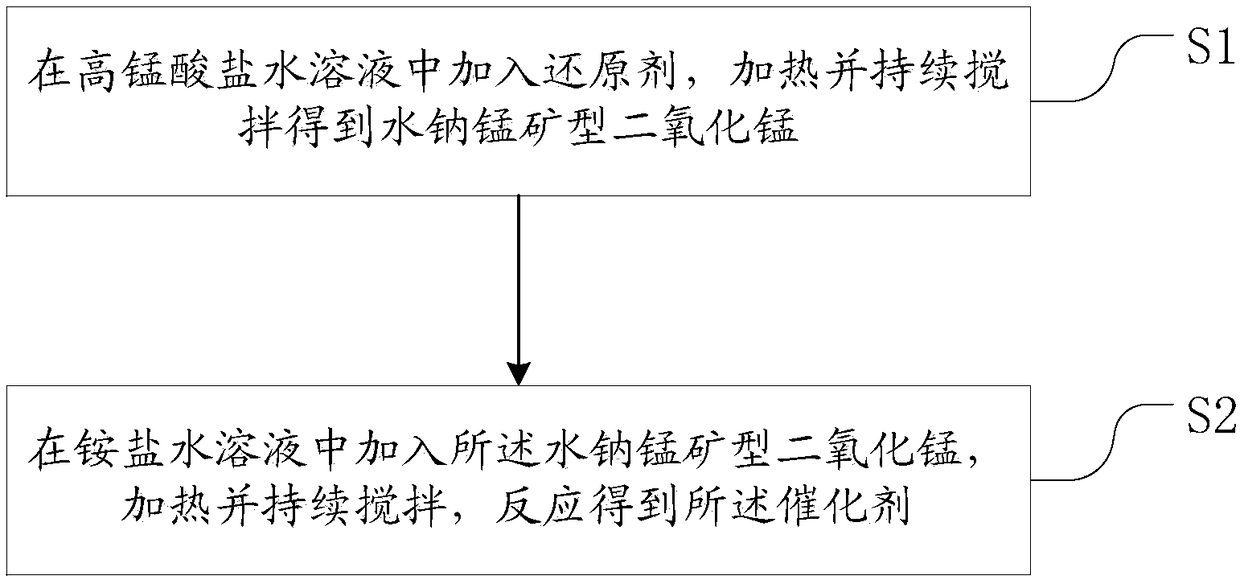
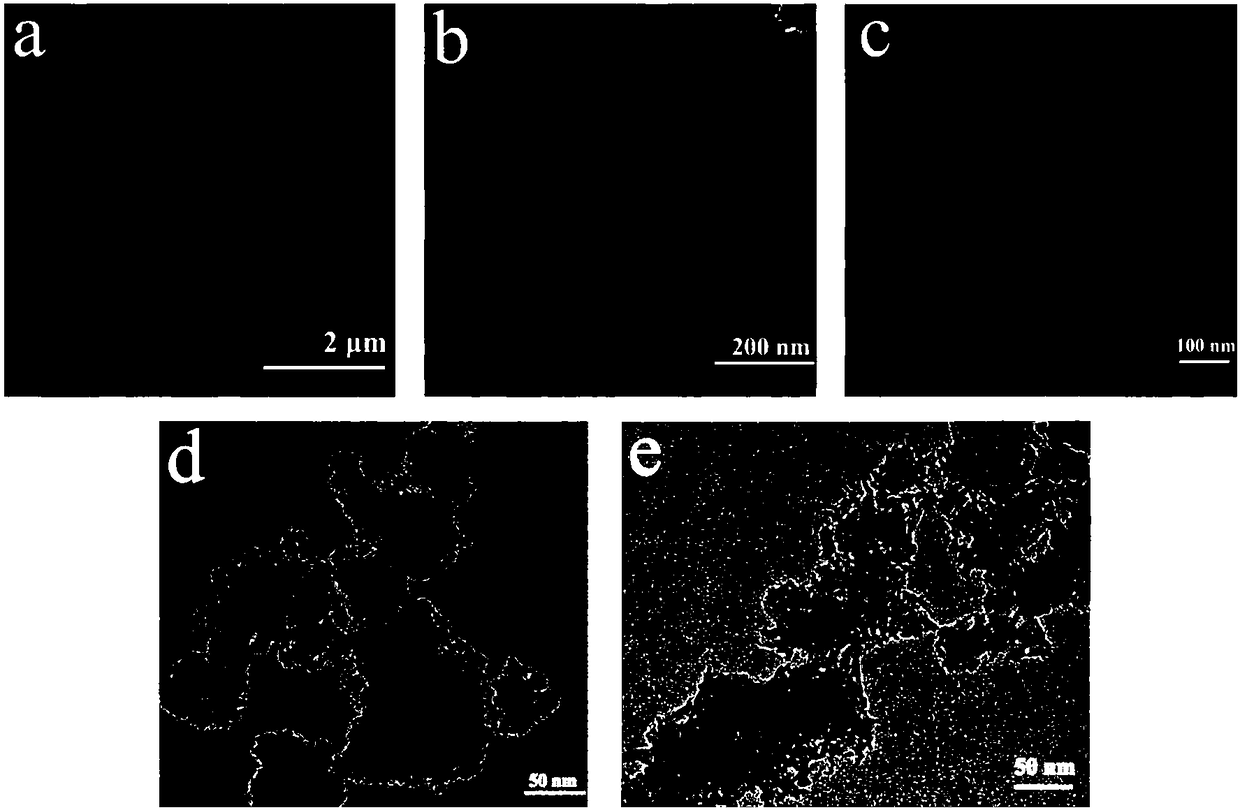
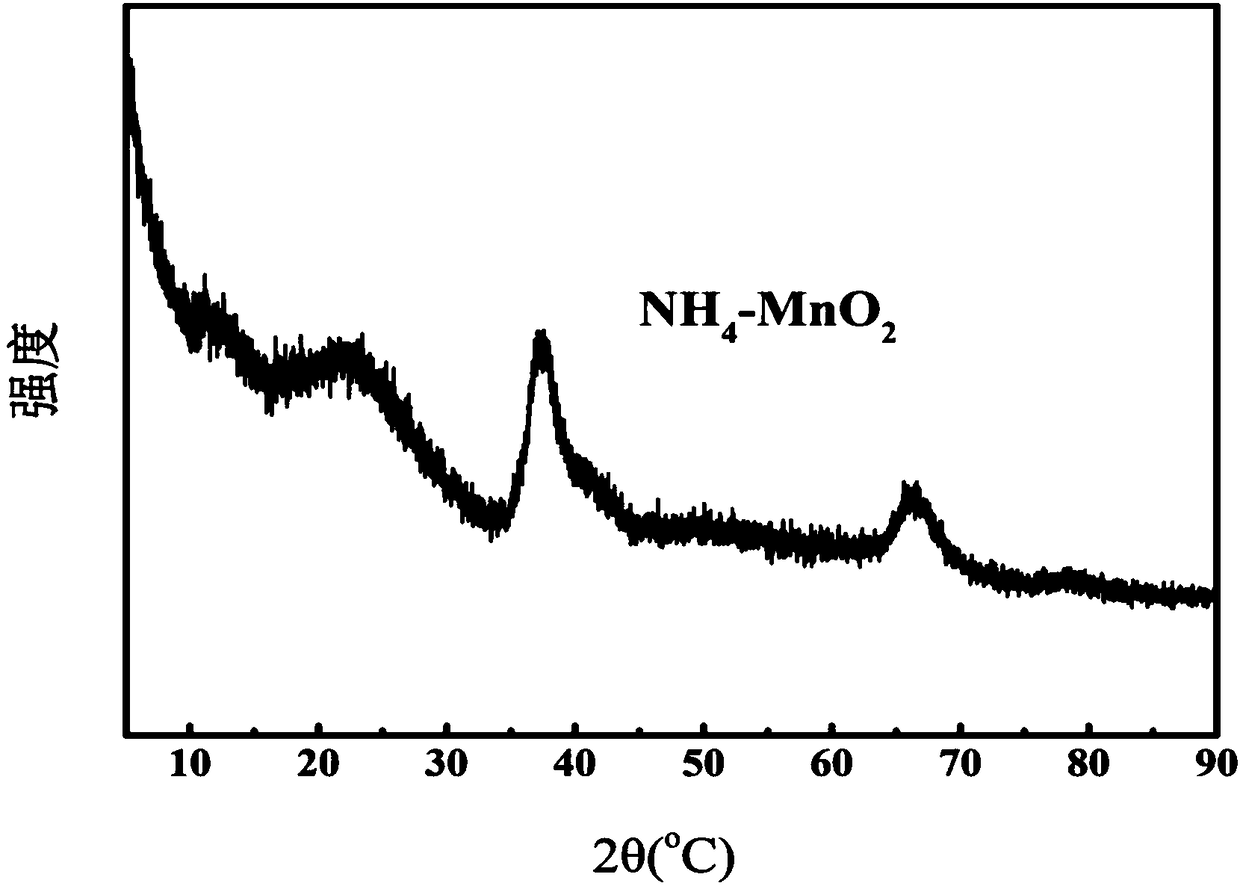
Preparation method of catalyst for ozone decomposition
An ozone decomposition and catalyst technology, which is applied in catalyst activation/preparation, separation methods, chemical instruments and methods, etc., and can solve problems such as short lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation method of the above-mentioned catalyst for ozonolysis uses ammonium salt to handle birnessite type manganese dioxide, due to NH 4 + The ions in the solution have a certain damage to the structure of the birnessite-type manganese dioxide formed, thereby increasing the specific surface area of the birnessite-type manganese dioxide catalyst and oxygen defects on the catalyst surface, and also increasing the surface acidity of the catalyst , thereby improving the catalytic activity of the birnessite-type manganese dioxide catalyst, and the prepared catalyst can still maintain a good catalytic effect on the ozone decomposition reaction in a humid environment. The preparation process does not require high temperature and high pressure, and has the advantages of simple preparation process and low cost The advantages are low cost, and the prepared catalyst can still maintain a good catalytic effect on the ozonolysis reaction in a humid environment.
[0039] I...
Embodiment 1
[0047] 3g KMnO 4 Add to a 250mL Erlenmeyer flask, add 100mL deionized water thereto, then place the Erlenmeyer flask into a constant temperature water bath at 40°C and magnetically stir the contents of the Erlenmeyer flask. Wait for KMnO 4 After the solid was completely dissolved, 15 g of CH was added 3 OH, continue magnetic stirring for 6h. After the reaction, the mixture in the Erlenmeyer flask was filtered and cleaned with deionized water to obtain a solid. The washed solid sample was put into an oven at 105°C for 12 hours to be heated and dried to obtain birnessite-type manganese dioxide.
[0048] Add 2g of dried birnessite-type manganese dioxide powder into a 100mL beaker, and add 40mL of 15g / L NH 4 Cl solution, the beaker was placed in a constant temperature water bath at 30°C and the mixture in the beaker was continuously magnetically stirred for 3h. After the reaction, the reacted mixture in the beaker was filtered and cleaned with deionized water to obtain a prod...
Embodiment 2
[0051] 3g NaMnO 4 Add it to a 250mL conical flask, then add 50mL deionized water therein, place the conical flask into a constant temperature water bath at 60°C and magnetically stir the contents in the conical flask. NaMnO 4 After the solids were completely dissolved, 30 g of CH 3 CH 2 OH, magnetically stirred for 12h. After the reaction, the mixture in the Erlenmeyer flask was filtered and cleaned with deionized water and filtered to obtain a solid. The washed solid sample was put into an oven at 105°C for 12 hours to be heated and dried to obtain birnessite-type manganese dioxide. The birnessite type manganese dioxide powder after 2g drying is added in the 100mL beaker, adds 40mL 60g / L thereinto (NH 4 ) 2 SO 4 solution, the beaker was placed in a constant temperature water bath at 50°C and the mixture in the beaker was continuously magnetically stirred for 8h. After the reaction, the reacted mixture in the beaker was filtered and washed with deionized water to obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com