Use of layered mesoporous birnessite type MnO2 cellular nano-sphere
A birnessite, honeycomb-shaped technology applied in the field of catalysts for the complete catalytic oxidation of formaldehyde
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
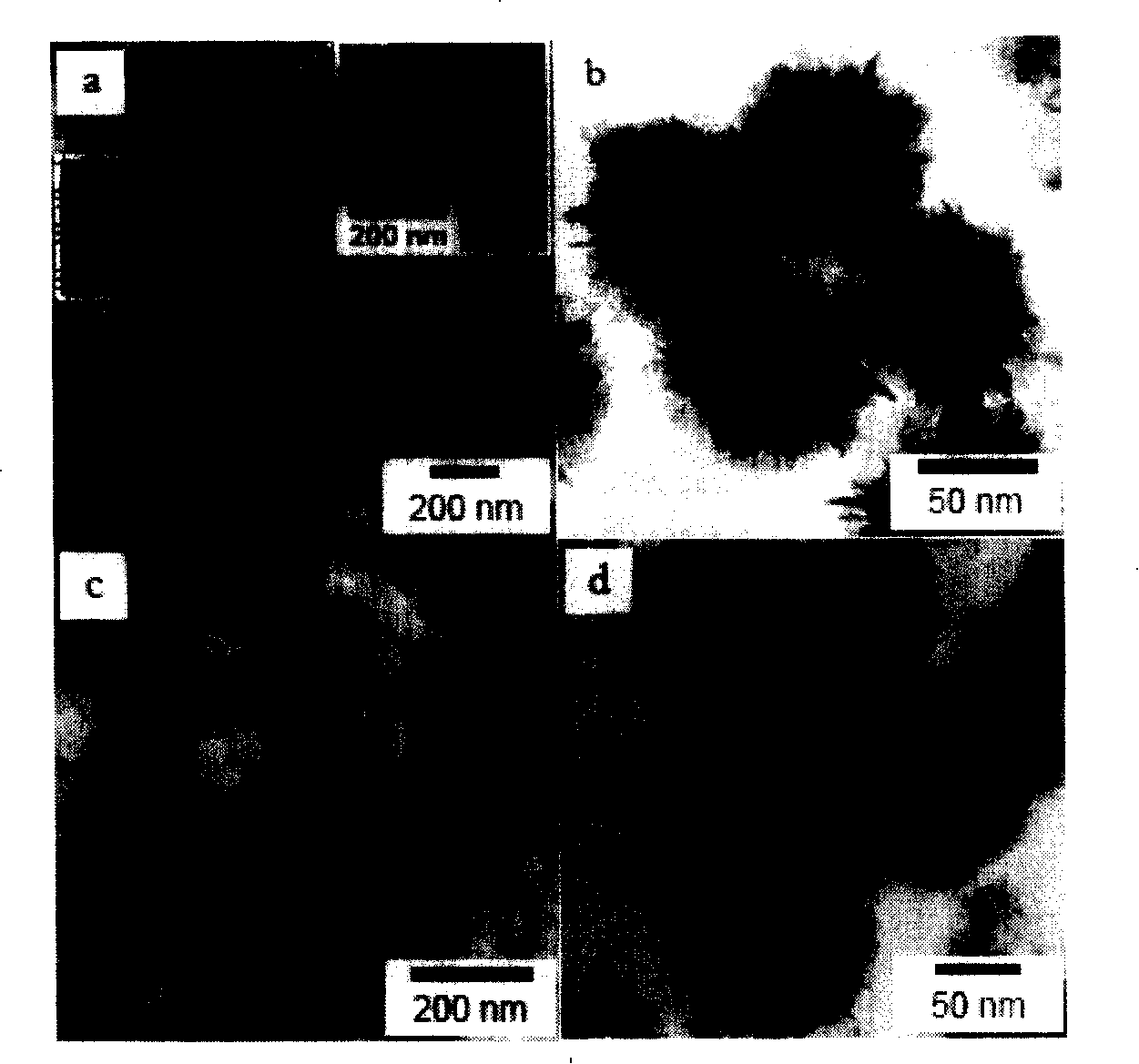
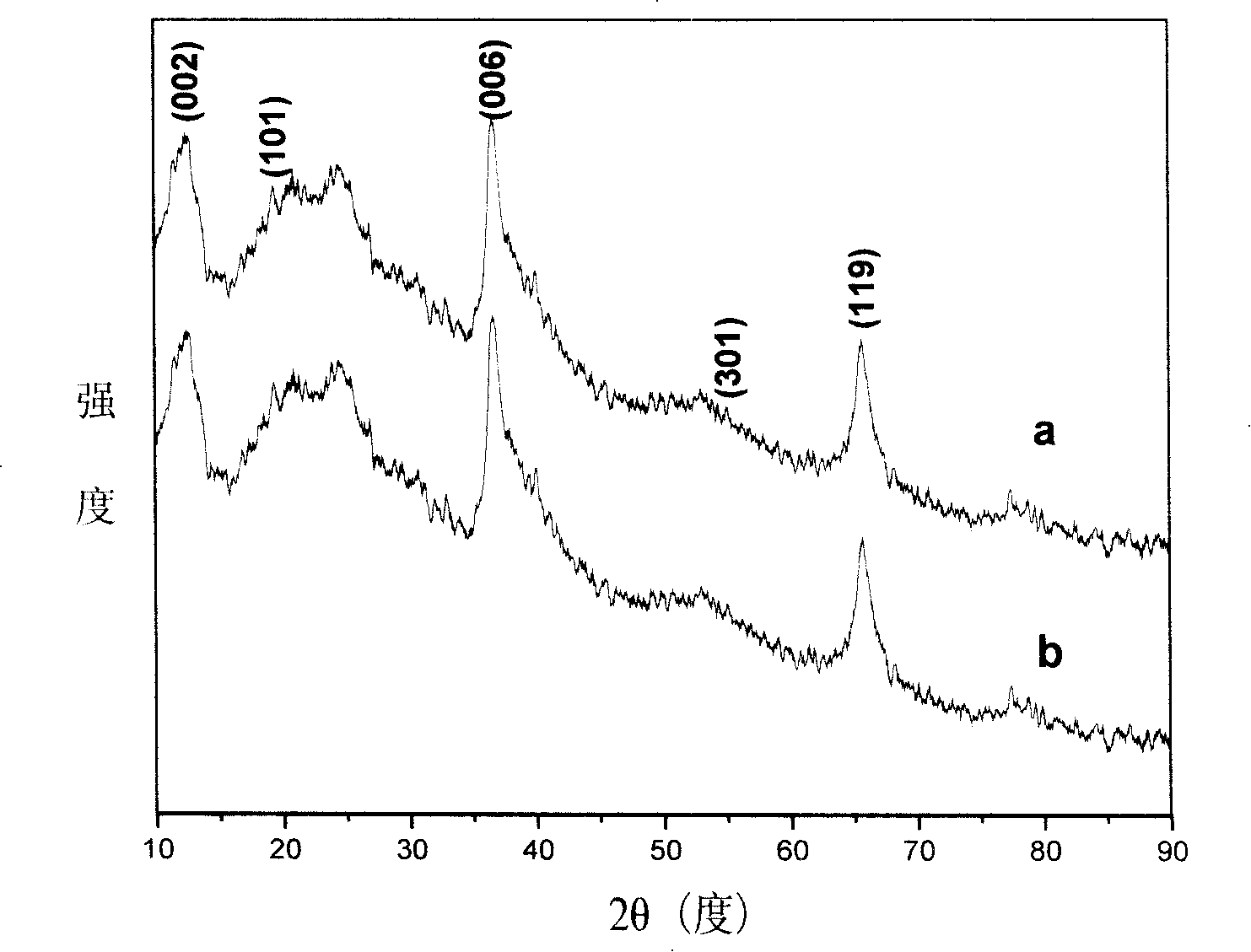
[0038] Take a certain amount of potassium permanganate with a purity of not less than 99.5% and dissolve it in 40-60 mL of distilled water, stir at room temperature for 15-30 minutes, and add 1 mL of oleic acid dropwise (the density of oleic acid is 0.891-0.899 g / mL, and the saponification value is not less than 3. The acid value is 190-200), the molar ratio of potassium permanganate / oleic acid is 1 / 10-3 / 10, and the stirring reaction is continued for not less than 5 hours. After reacting for a certain period of time until solid precipitates appear, separate at 4000 rpm for 10 to 15 minutes, then wash the sample with distilled water and ethanol, and repeat 3 to 5 times. The final sample was vacuum-dried at 50-70 degrees Celsius for more than 10 hours, and the obtained dark brown layered mesoporous birnessite-type MnO 2 Honeycomb nanospheres such as figure 1 shown in a and 1b. Take a small amount of dried sample and redisperse it in ethanol, ultrasonically disperse it under 1...
Embodiment 2
[0042] Prepare layered mesoporous birnessite type MnO according to the method of Example 1 2 Honeycomb hollow nanosphere structure. Take a certain amount of potassium permanganate with a purity of not less than 99.5% and dissolve it in 40-60 mL of distilled water, stir at room temperature for 15-30 minutes, and add 1 mL of oleic acid dropwise (the density of oleic acid is 0.891-0.899 g / mL, and the saponification value is not less than 3. The acid value is 190-200), the molar ratio of potassium permanganate / oleic acid is 1-2, and the stirring reaction is continued for not less than 5 hours. After reacting for a certain period of time, separate at 4000 r / min for 10-15 minutes, then wash the sample with distilled water and ethanol, and repeat 3-5 times. The final prepared samples were dispersed and dried in vacuum at 50-70 degrees Celsius for more than 10 hours, and the obtained dark brown layered mesoporous birnessite-type MnO 2 Honeycomb hollow nanospheres such as figure 1 ...
Embodiment 3
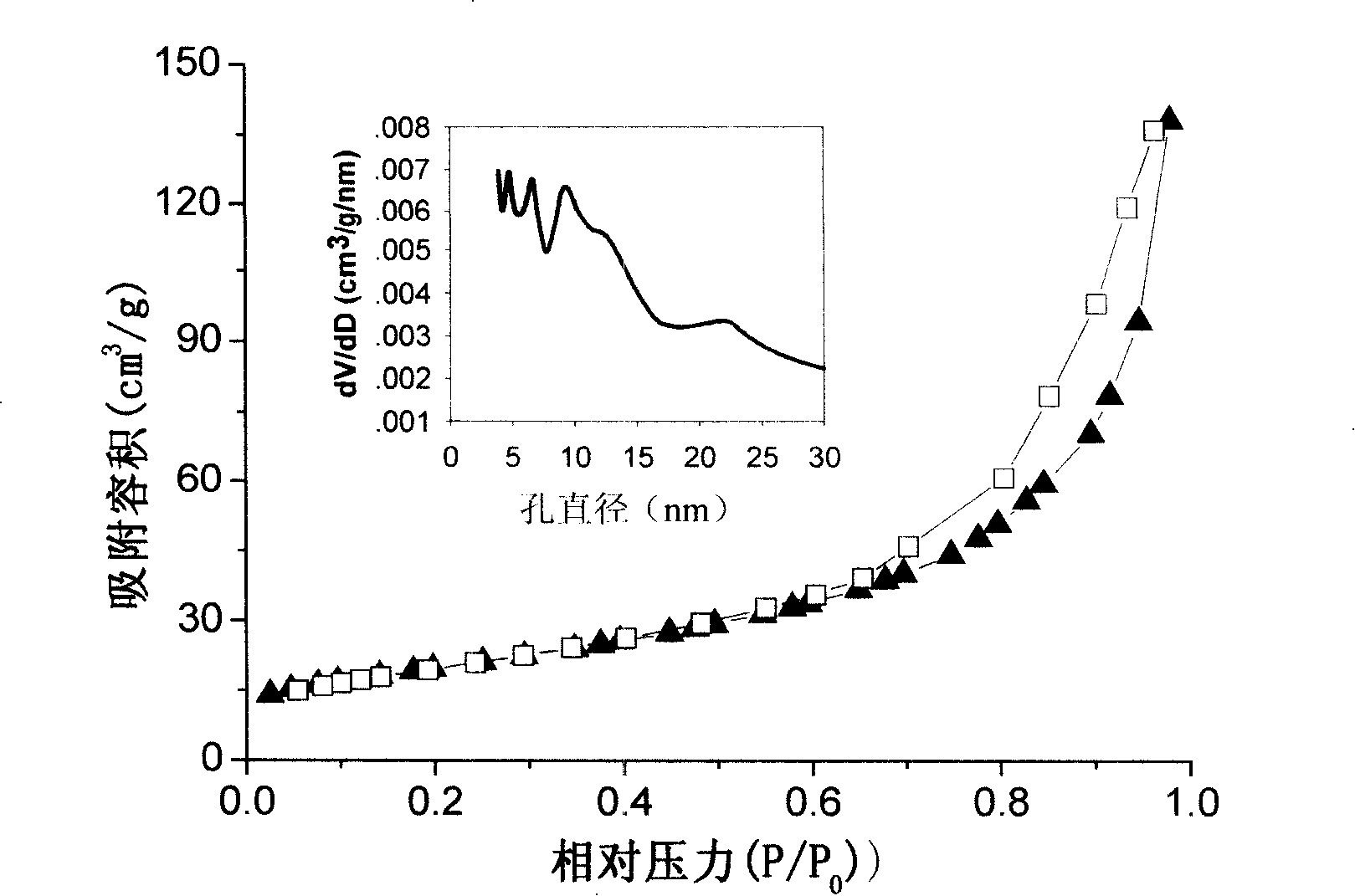
[0046] Get the layered mesoporous birnessite type MnO obtained in embodiment 1 and 2 2 Honeycomb nanospheres and honeycomb hollow nanospheres were degassed at 150 degrees Celsius respectively, and their nitrogen adsorption characteristics at -196 degrees Celsius were measured on a nitrogen adsorption-desorption analyzer (Quantachrome NOVA4200e, Quanta, USA). The experimental results are shown in the curve in Figure 3, which shows that the obtained layered mesoporous birnessite-type MnO 2 Honeycomb nanospheres and honeycomb hollow nanospheres have good gas adsorption properties. Figure 3a layered mesoporous birnessite-type MnO 2 Nitrogen adsorption-desorption isotherms and pore size distribution curves of honeycomb nanospheres; Figure 3b layered mesoporous birnessite-type MnO 2 Nitrogen adsorption-desorption isotherms and pore size distribution curves of honeycomb hollow nanospheres. The isotherm has a hysteresis loop, indicating that the as-prepared layered mesoporous bi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com