Method for asymmetrically synthesizing chiral olefinic acid ester
A synthesis method and alkenoate technology, applied in the field of medicine, can solve the problems of few synthesis methods and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
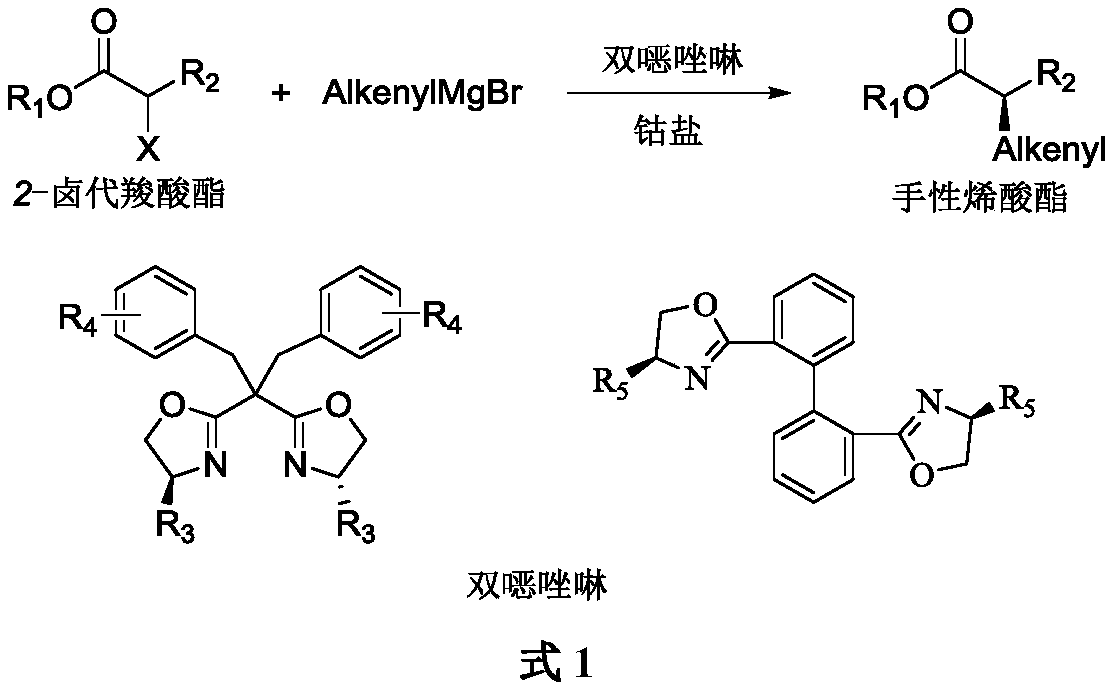
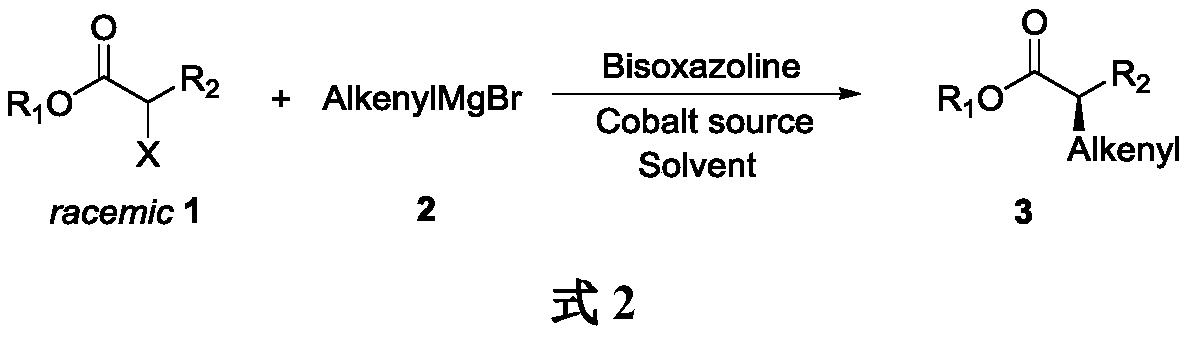
[0019] Synthesis of (S)-2,4-dimethyl-3-pentenoic acid benzyl ester 3a
[0020] Add anhydrous CoCl to a dry Shrek reaction flask 2 (6.5mg, 0.05mmol), pass through argon, dry in vacuum for 2h, add bis The oxazoline chiral ligand L1 (37.6mg, 0.06mmol) and anhydrous THF (3mL), stirred at room temperature for 2h, then lowered the reaction temperature to -40°C, added benzyl 2-bromopropionate (60.8mg, 0.25 mmol), slowly dropwise added 2-methyl-1-propenylmagnesium bromide (2.7mL, 0.37M tetrahydrofuran solution, 1.0mmol), continued to stir the reaction at -40°C for 5h, added saturated aqueous ammonium chloride solution (3mL) Quenches the reaction. Separate the layers, extract the aqueous layer with diethyl ether (10mL×4), combine the organic layers, wash with saturated aqueous sodium chloride (10mL), dry over anhydrous sodium sulfate, concentrate under reduced pressure and purify by silica gel column chromatography (n-hexane / ethyl acetate 80:1), to obtain pale yellow oil 3a (43.7mg...
Embodiment 2
[0022] Synthesis of (S)-2-methyl-3-butene benzyl ester 4a
[0023] Add anhydrous LiI (26.8 mg, 0.2 mmol) and CoCl to a dry Shrek reaction flask 2 (13.0mg, 0.1mmol), pass through argon, dry in vacuum for 2h, add bis The oxazoline chiral ligand L2 (56.7 mg, 0.12 mmol) and anhydrous THF (3 mL) were stirred at room temperature for 2 h. The reaction temperature was lowered to -20°C, benzyl 2-bromopropionate (60.8mg, 0.25mmol) was added, vinylmagnesium bromide (2.1mL, 0.6M solution in tetrahydrofuran, 1.25mmol) was slowly added dropwise with stirring, and in- The stirring reaction was continued at 20°C for 24h. Add saturated aqueous ammonium chloride solution (3mL) to quench the reaction, separate the layers, extract the aqueous layer with ether (10mL×4), combine the organic layers, wash with saturated aqueous sodium chloride solution (10mL), dry over anhydrous sodium sulfate, and concentrate under reduced pressure Afterwards, it was purified by silica gel column chromatography ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap