Boron-nitrogen structured multi-aromatic-ring flame-retardant epoxy monomer as well as preparation method and application thereof
A technology of epoxy monomer and aromatic ring, which is applied in the field of flame-retardant epoxy resin with boron-nitrogen structure polyaromatic ring and its preparation, can solve the problems of poor flame retardancy, achieve improved flame retardancy, simple reaction process, and improved cross-fertilization The effect of link density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
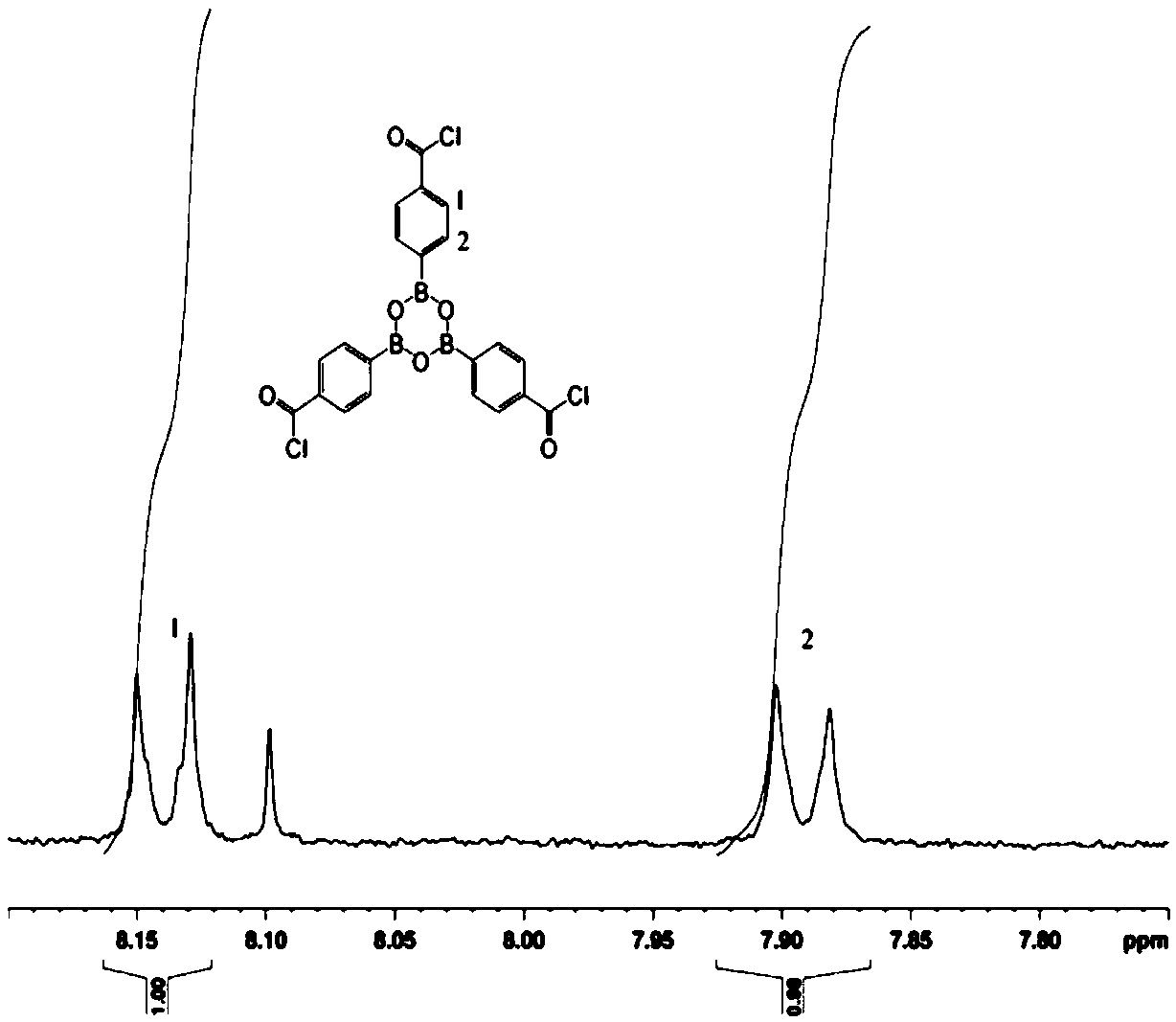
[0037] (1) In a three-necked flask equipped with a nitrogen protection device, add 0.06mol 4-carboxyphenylboronic acid and 0.06mol thionyl chloride, stir at room temperature for 10min, raise the temperature to 60°C, and react for 10h. During the reaction, the reaction system is kept at in a nitrogen atmosphere. Thionyl chloride and the water produced by the reaction were distilled off under reduced pressure to obtain a white solid, which was dried in vacuum at 50°C for 3 hours to obtain a white powdery solid 4,4′,4″-(boroxine-2,4,6) - Tribenzoyl chloride.
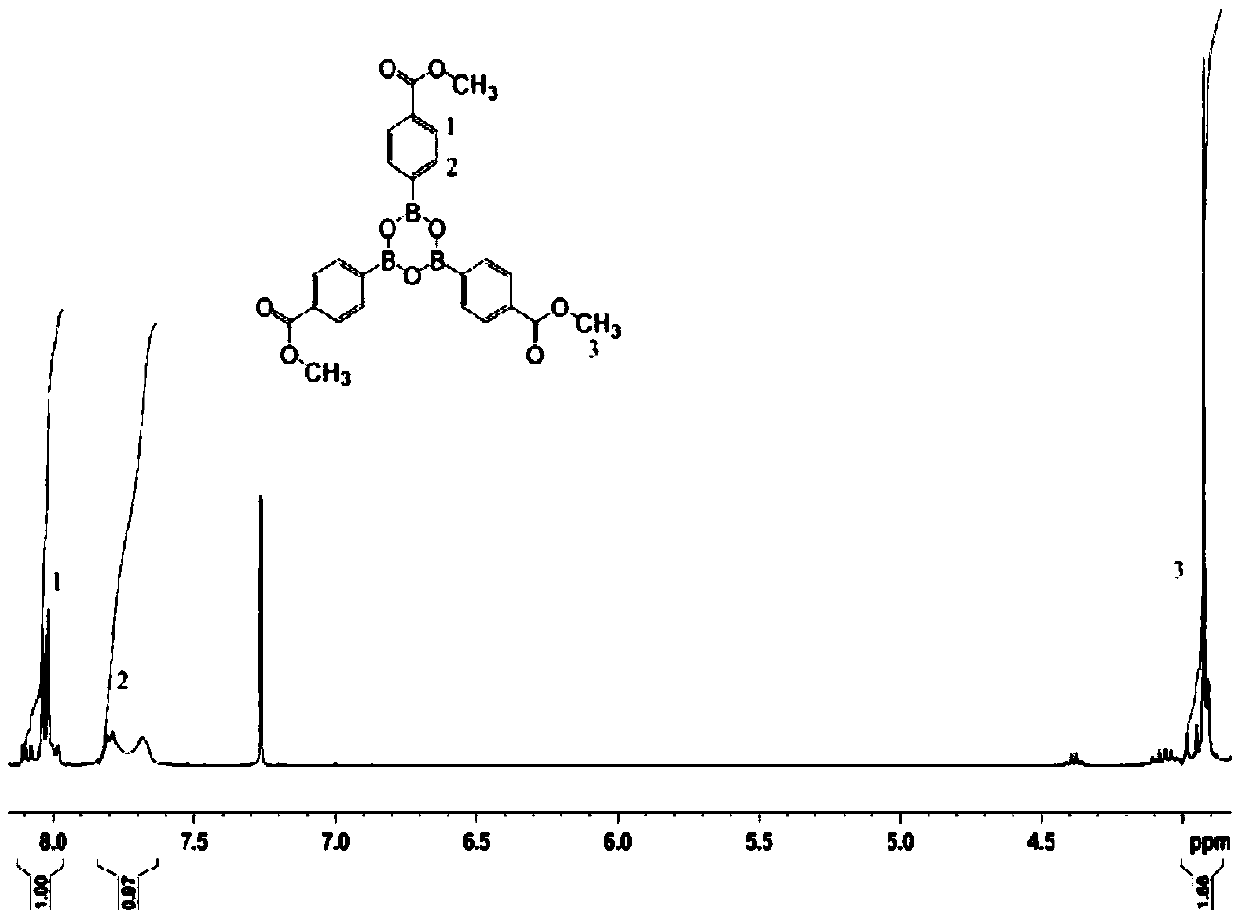
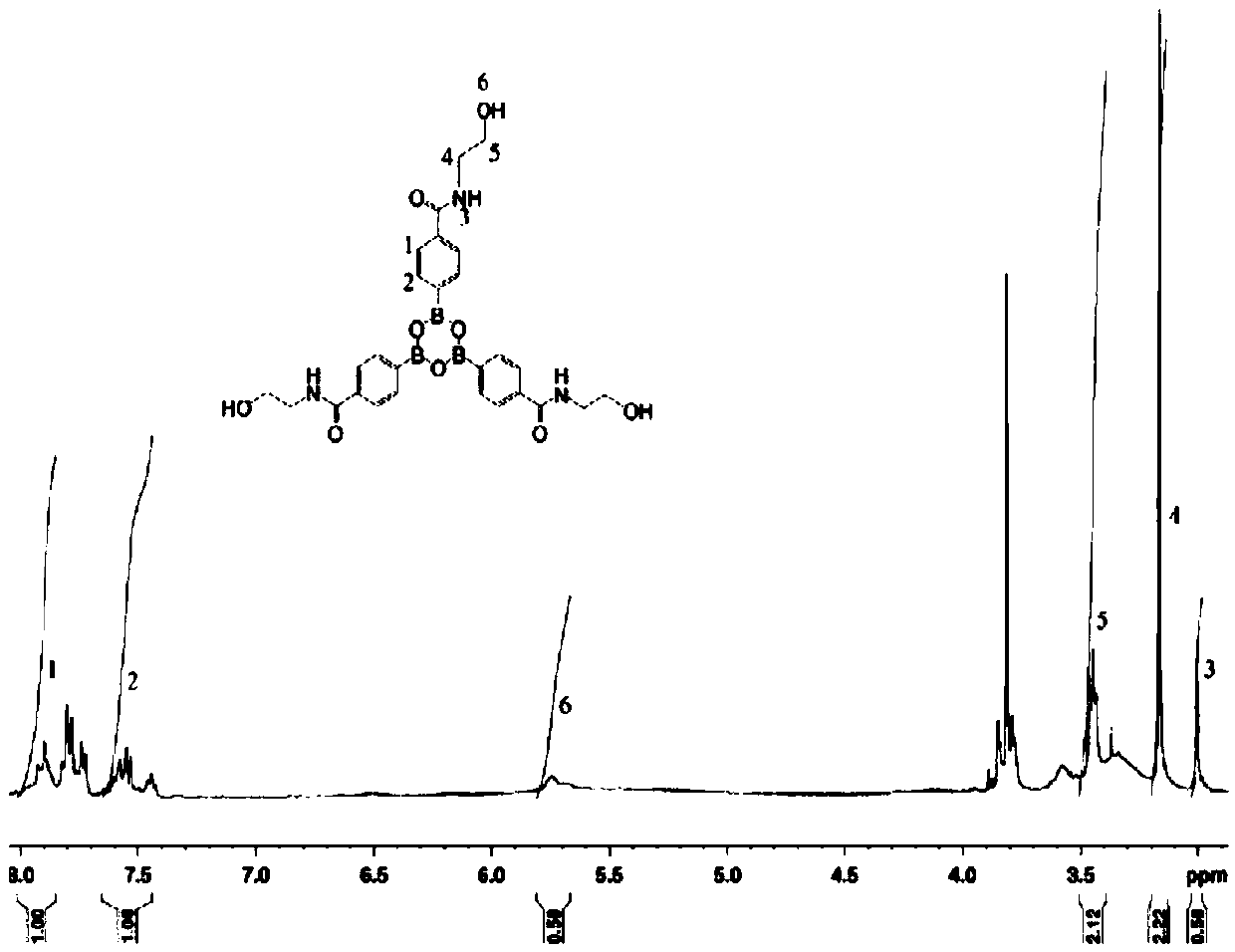
[0038] (2) Add 0.016mol 4,4′,4″-(boroxine-2,4,6)-tribenzoyl chloride and 50ml acetone to a four-necked flask equipped with a nitrogen protection device, and stir at room temperature for 10min ; Weigh 0.048mol of methanol and place it in a constant pressure dropping funnel, add it dropwise to the flask under stirring conditions, and the dropping time is controlled at 20min. After the methanol is added dropwise, stir at room...
Embodiment 2
[0046] (1) In a three-necked flask equipped with a nitrogen protection device, add 0.06mol 4-carboxyphenylboronic acid and 0.06mol thionyl chloride, stir at room temperature for 60min, raise the temperature to 90°C, and react for 20h. During the reaction, the reaction system is kept at in a nitrogen atmosphere. Thionyl chloride and the water produced by the reaction were distilled off under reduced pressure to obtain a white solid, which was dried in vacuum at 80°C for 6 hours to obtain a white powdery solid 4,4′,4″-(boroxine-2,4,6) - Tribenzoyl chloride.
[0047] (2) In a four-necked flask equipped with a nitrogen protection device, add 0.016mol 4,4′,4″-(boroxine-2,4,6)-tribenzoyl chloride and 120ml tetrahydrofuran, and stir at room temperature for 60min Weigh 0.048mol of methanol and place it in a constant pressure dropping funnel, add it dropwise to the flask under stirring, and the dropping time is controlled at 90min. After the dropwise addition of methanol is completed,...
Embodiment 3
[0055] (1) In a three-necked flask equipped with a nitrogen protection device, add 0.06mol 4-carboxyphenylboronic acid and 0.06mol thionyl chloride, stir at room temperature for 40min, raise the temperature to 70°C, and react for 13h. During the reaction, the reaction system is kept at in a nitrogen atmosphere. Thionyl chloride and the water produced by the reaction were distilled off under reduced pressure to obtain a white solid, which was dried in vacuum at 70°C for 5 hours to obtain a white powdery solid 4,4′,4″-(boroxine-2,4,6) - Tribenzoyl chloride.
[0056] (2) In a four-necked flask equipped with a nitrogen protection device, add 0.016mol 4,4′,4″-(boroxine-2,4,6)-tribenzoyl chloride and 70ml of dichloromethane, at room temperature Stir for 30 minutes; weigh 0.048 mol of methanol and place it in a constant pressure dropping funnel, add it dropwise to the flask under stirring conditions, and the dropping time is controlled at 70 minutes. After the dropwise addition of m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| bending strength | aaaaa | aaaaa |
| impact strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com