Medicine composition for treating non-hodgkin lymphoma
A non-Hodgkin, lymphoma technology, used in drug combinations, anti-tumor drugs, pharmaceutical formulations, etc., can solve the problem of no clinical trial reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Construction of Rituximab-mediated CDC-tolerant Raji cells and Ramos cells
[0056] Rituximab-mediated ADCC is carried out by immune cells of individual patients, and their resistance to ADCC is caused by intrinsic characteristics of immune cells, such as FcγRIIIa gene polymorphisms. However, apoptosis plays only a negligible role in the antitumor activity of rituximab. Therefore, we constructed two BL cell lines, Ramos640 and Raji32, that were resistant to rituximab-mediated complement-dependent cytotoxicity at rituximab concentrations of 640 and 32 μg / mL, respectively. Its construction method is as follows:
[0057]Two BL cell lines, Raji and Ramos, were purchased from American typeculture collection (ATCC) (Manassas, VA), and the cells were cultured in 10% (volume content) fetal bovine serum (GIBCO BRL Company, Grand Island, NY) and 1% (volume content) penicillin / streptomycin (Ambion, Austin, TX) in RPMI 1640 medium.
[0058] As a complement resource, no...
Embodiment 2
[0060] Example 2 Western blot analysis and CDC effect determination
[0061] The present invention performed immunoblot analysis according to standard methods.
[0062] The CDC effect was determined by fluorescence-activated cell sorting (FACS) analysis to detect propidium iodide (PI)-stained positive cells. Specifically, after washing with PBS, cells were incubated with fluorescein-conjugated antibodies for 30 minutes, then rinsed and resuspended in PBS. Flow cytometric analysis was performed on a Cytomics FC500MPL flow cytometer (Beckman Coulter, Brea, CA) and analyzed with FlowJo software (Ashland, OR). We sorted cells based on the relative fluorescence with the MoFlo XDP instrument (Beckman Coulter, Brea, CA) using the PE Annexin V Apoptosis Detection Kit (BD Pharmingen, San Diego, CA) according to its manufacturer's instructions Apoptosis analysis.
Embodiment 3
[0063] Example 3 Down-regulation of CD20 and up-regulation of CD59 lead to tolerance of BL cells to rituximab-mediated CDC
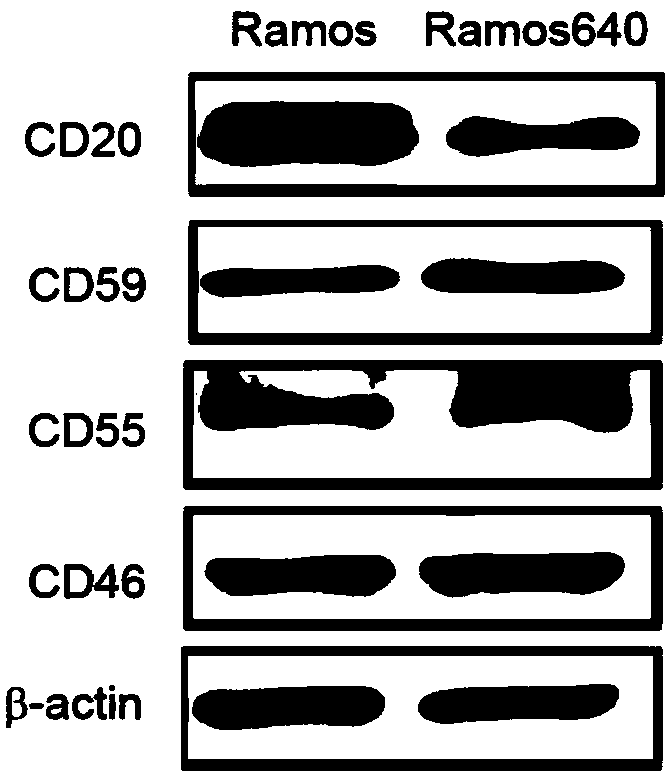
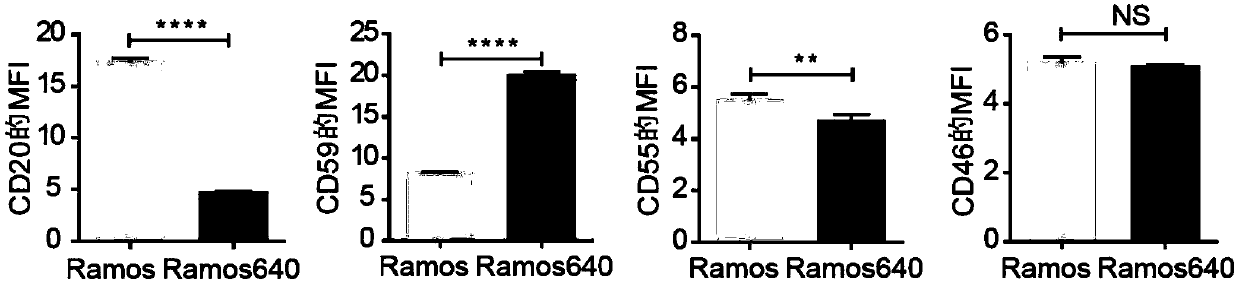
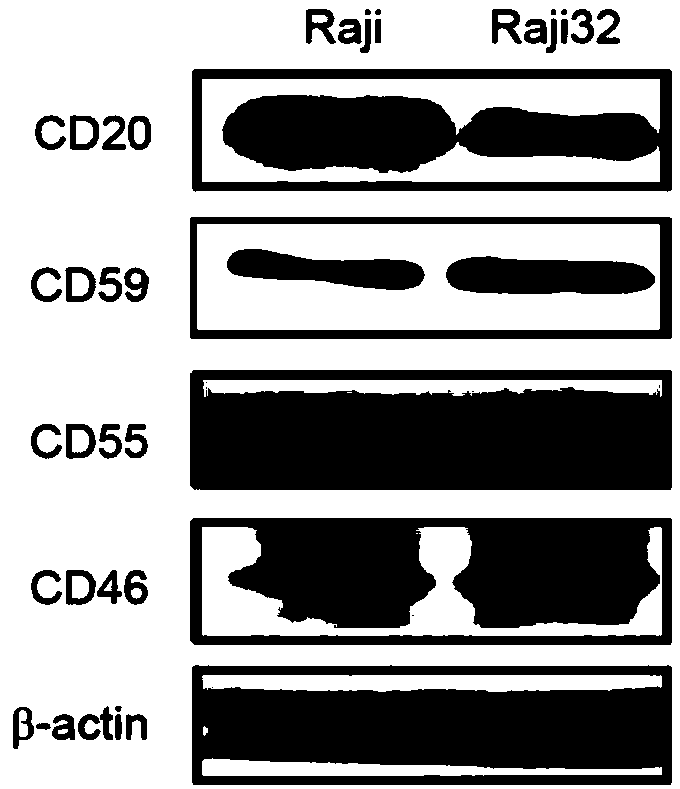
[0064] Using Western blot analysis ( figure 1 and image 3 ) and FACS ( figure 2 and Figure 4 ), we found that the expression of CD20 was decreased and the expression of CD59 was increased in both drug-resistant cells compared with their original cells ( Figure 1-Figure 4 ). However, the expression of two other membrane complement regulatory proteins (mCRP), CD55 and CD46, was not consistent in the two resistant cells. The expression of CD55 decreased in Ramos640, but increased in Raji32; while the expression of CD46 did not change in Ramos640, but decreased in Raji32 ( Figure 1-Figure 4 ). These results are consistent with previous reports that decreased CD20 expression and elevated CD59 expression lead to resistance of BL cells to rituximab-mediated CDC. However, since the previous studies did not obtain good results in the studies on increa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com