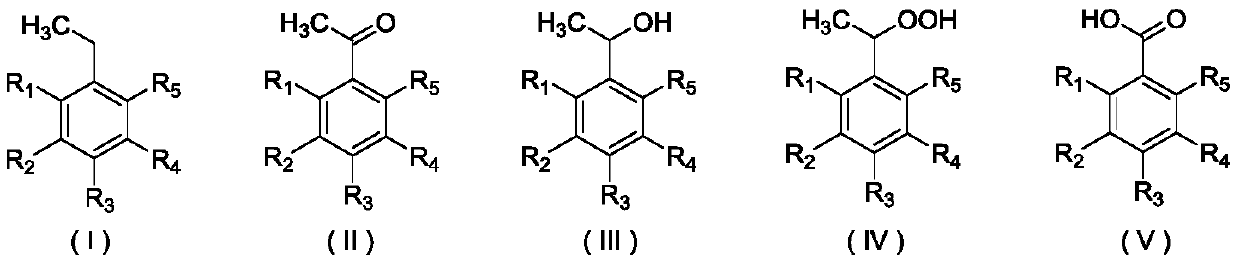
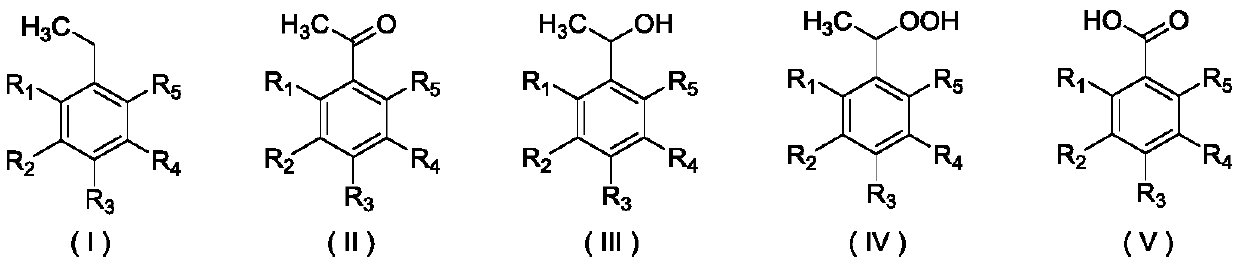
Method for preparing acetophenone and its derivatives through selectively oxidizing ethylbenzene and its derivatives
A derivative, the technology of acetophenone, applied in the field of organic chemical industry and fine organic synthesis, can solve the problems of incomplete decomposition of peroxides, uneasy control of the reaction, high reaction temperature, etc., and achieve simple oxidation transformation, novel reaction mode, selective sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a 100mL agate ball mill jar, 1.51g (10mmol) 4-nitroethylbenzene, 0.0009g (0.0050mmol) cobalt acetate, 2.57g (20mmol) 70% t-butyl hydroperoxide aqueous solution and 4.53g anhydrous sulfuric acid Mix the sodium evenly, and seal the ball mill jar. At room temperature, the ball milling reaction was carried out at 600 rpm for 12.0 h, and the ball milling was stopped every 1.0 h to release the gas in the ball milling tank. After the reaction was completed, the resulting reaction mixture was dissolved in 30 mL of absolute ethanol, and stirred at room temperature for 30.0 min. Filter, wash the obtained filter cake with 2×10 mL of absolute ethanol, combine the ethanol solution, and dilute the obtained ethanol solution to 100 mL. Pipette 10 mL of the resulting solution, add internal standard 2-naphthoic acid, and perform liquid chromatography analysis. The conversion rate of 4-nitroethylbenzene was 27%, the selectivity of 4-nitroacetophenone was 92%, the selectivity of 1-(4-...
Embodiment 2
[0025] In a 100mL agate ball mill jar, 1.51g (10mmol) 4-nitroethylbenzene, 0.0044g (0.0250mmol) cobalt acetate, 2.57g (20mmol) 70% t-butyl hydroperoxide aqueous solution and 4.53g anhydrous sulfuric acid Mix the sodium evenly, and seal the ball mill jar. At room temperature, the ball milling reaction was carried out at 600 rpm for 12.0 h, and the ball milling was stopped every 1.0 h to release the gas in the ball milling tank. After the reaction was completed, the resulting reaction mixture was dissolved in 30 mL of absolute ethanol, and stirred at room temperature for 20.0 min. Filter, wash the obtained filter cake with 2×10 mL of absolute ethanol, combine the ethanol solution, and dilute the obtained ethanol solution to 100 mL. Pipette 10 mL of the resulting solution, add internal standard 2-naphthoic acid, and perform liquid chromatography analysis. The conversion rate of 4-nitroethylbenzene was 30%, the selectivity of 4-nitroacetophenone was 97%, the selectivity of 1-(4-...
Embodiment 3
[0027] In a 100mL agate ball mill jar, 1.51g (10mmol) 4-nitroethylbenzene, 0.0018g (0.0100mmol) cobalt acetate, 2.57g (20mmol) 70% t-butyl hydroperoxide aqueous solution and 4.53g anhydrous sulfuric acid Mix the sodium evenly, and seal the ball mill jar. At room temperature, the ball milling reaction was carried out at 600 rpm for 12.0 h, and the ball milling was stopped every 1.0 h to release the gas in the ball milling tank. After the reaction was completed, the resulting reaction mixture was dissolved in 30 mL of absolute ethanol, and stirred at room temperature for 50.0 min. Filter, wash the obtained filter cake with 2×10 mL of absolute ethanol, combine the ethanol solution, and dilute the obtained ethanol solution to 100 mL. Pipette 10 mL of the resulting solution, add internal standard 2-naphthoic acid, and perform liquid chromatography analysis. The conversion rate of 4-nitroethylbenzene was 28%, the selectivity of 4-nitroacetophenone was 96%, the selectivity of 1-(4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com