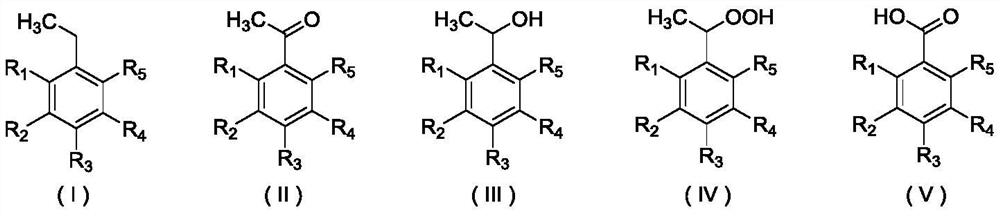
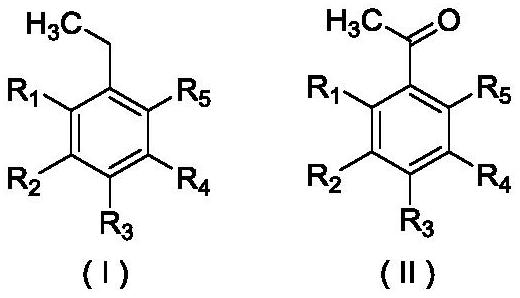
A method for preparing acetophenone and its derivatives by selective oxidation of ethylbenzene and its derivatives
A derivative, the technology of acetophenone, applied in the field of organic chemical industry and fine organic synthesis, can solve the problems of incomplete decomposition of peroxides, uneasy control of the reaction, high reaction temperature, etc., and achieve simple oxidation transformation, novel reaction mode, selective sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 100 mL agate ball mill jar, mix 1.51 g (10 mmol) 4-nitroethylbenzene, 0.0009 g (0.0050 mmol) cobalt acetate, 2.57 g (20 mmol) 70% t-butyl hydroperoxide aqueous solution and 4.53 g anhydrous sulfuric acid Mix the sodium well and seal the ball mill jar. At room temperature, the ball milling reaction was carried out at 600 rpm for 12.0 h, and the ball milling was stopped every 1.0 h, and the gas in the ball milling jar was released. After the reaction was completed, the obtained reaction mixture was dissolved in 30 mL of absolute ethanol, and stirred at room temperature for 30.0 min. Filter, wash the obtained filter cake with 2×10 mL of absolute ethanol, combine the ethanol solutions, and dilute the obtained ethanol solution to 100 mL. Pipette 10 mL of the obtained solution, add internal standard 2-naphthoic acid, and carry out liquid chromatography analysis. The conversion rate of 4-nitroethylbenzene was 27%, the selectivity of 4-nitroacetophenone was 92%, the sele...
Embodiment 2
[0021] In a 100 mL agate ball mill jar, mix 1.51 g (10 mmol) 4-nitroethylbenzene, 0.0044 g (0.0250 mmol) cobalt acetate, 2.57 g (20 mmol) 70% t-butyl hydroperoxide aqueous solution and 4.53 g anhydrous sulfuric acid Mix the sodium well and seal the ball mill jar. At room temperature, the ball milling reaction was carried out at 600 rpm for 12.0 h, and the ball milling was stopped every 1.0 h, and the gas in the ball milling jar was released. After the reaction was completed, the obtained reaction mixture was dissolved in 30 mL of absolute ethanol, and stirred at room temperature for 20.0 min. Filter, wash the obtained filter cake with 2×10 mL of absolute ethanol, combine the ethanol solutions, and dilute the obtained ethanol solution to 100 mL. Pipette 10 mL of the obtained solution, add internal standard 2-naphthoic acid, and carry out liquid chromatography analysis. The conversion rate of 4-nitroethylbenzene was 30%, the selectivity of 4-nitroacetophenone was 97%, the sele...
Embodiment 3
[0023] In a 100 mL agate ball mill jar, mix 1.51 g (10 mmol) 4-nitroethylbenzene, 0.0018 g (0.0100 mmol) cobalt acetate, 2.57 g (20 mmol) 70% t-butyl hydroperoxide aqueous solution and 4.53 g anhydrous sulfuric acid Mix the sodium well and seal the ball mill jar. At room temperature, the ball milling reaction was carried out at 600 rpm for 12.0 h, and the ball milling was stopped every 1.0 h, and the gas in the ball milling jar was released. After the reaction was completed, the obtained reaction mixture was dissolved in 30 mL of absolute ethanol, and stirred at room temperature for 50.0 min. Filter, wash the obtained filter cake with 2×10 mL of absolute ethanol, combine the ethanol solutions, and dilute the obtained ethanol solution to 100 mL. Pipette 10 mL of the obtained solution, add internal standard 2-naphthoic acid, and carry out liquid chromatography analysis. The conversion rate of 4-nitroethylbenzene was 28%, the selectivity of 4-nitroacetophenone was 96%, the sele...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com