Green preparation method of isothiocyanate compounds
A technology of isothiocyanate and compounds, which is applied in the field of green preparation of isothiocyanate compounds, can solve the problems of difficult solid waste treatment, incomplete dissolution, and reduced product content, so as to avoid post-processing work, low cost, and easy The effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
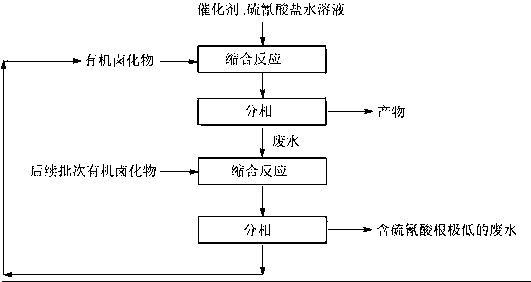
[0029] Install a stirrer, a reflux condenser, and a thermometer on a 1000mL four-necked reaction flask, add 496g (1.2mol) 20% sodium thiocyanate aqueous solution and 0.3g N,N-dimethyl-4-aminopyridine into the four-necked flask for 4 hours 77.3g of 3-chloropropene (1.0mol) was added dropwise at 60°C, and the reaction was kept for 1 hour after the dropwise addition. After standing still, the phases were separated, and the organic phase was 100 g (99.2%) of 3-isothiocyanatopropyl-1-ene, with a yield of 99.1% (calculated as 3-chloropropene). The water phase was added to the flask, and 77.3g of 3-chloropropene (1.0mol) was added dropwise at 60°C within 2 hours, and the phases were separated after standing. The organic phase was a mixture of 3-isothiocyanatopropyl-1-ene and chloropropene 81.5 g, the concentration of thiocyanate in the wastewater is 4ppm, and the COD is 1814mg / L. Then add 413g (1.0mol) 20% sodium thiocyanate aqueous solution and 0.3g N,N-dimethyl-4-aminopyridine int...
Embodiment 2
[0031] Install a stirrer, a reflux condenser, and a thermometer on a 1000mL four-necked reaction flask, add 356g (1.1mol) 20% potassium thiocyanate aqueous solution and 0.2g N,N-dimethyl-4-aminopyridine into the four-necked flask for 4 hours 138.5 g of bromobutane (1.0 mol) was added dropwise at 98°C, and the reaction was kept for 1 hour after the dropwise addition was completed. After standing still, the phases were separated, and the organic phase was 115.9 g (98.8%) of 1-isothiocyanatobutane, with a yield of 99.5% (calculated as bromobutane). Add the water phase to the flask, add 138.5g bromobutane (1.0mol) dropwise at 98°C within 2 hours, stand still and separate the phases, the organic phase is a mixture of 1-isothiocyanatobutane and bromobutane 136.2g, in the waste water Thiocyanate concentration 5ppm, COD 1927mg / L. Then add 323.7g (1.0mol) 20% aqueous solution of potassium thiocyanate and 0.2g N,N-dimethyl-4-aminopyridine into the four-necked flask, and dropwise add th...
Embodiment 3
[0033] Install a stirrer, a reflux condenser, and a thermometer on a 1000mL four-necked reaction flask, add 456g (1.2mol) 20% ammonium thiocyanate aqueous solution and 0.3g N,N-dimethyl-4-aminopyridine to the four-necked flask for 4 hours 122.3g of 3-bromopropene (1.0mol) was added dropwise at 50°C, and the reaction was kept for 1 hour after the dropwise addition. After standing still, the phases were separated, and the organic phase was 99.2 g (99.0%) of 3-isothiocyanatopropyl-1-ene, with a yield of 99.2% (calculated as 3-bromopropene). Add the water phase to the flask, add 122.3g of 3-bromopropene (1.0mol) dropwise at 50°C within 2 hours, let stand to separate the phases, and the organic phase is a mixture of 3-isothiocyanatopropyl-1-ene and 3-bromopropene Mixture 117.0g, concentration of thiocyanate in wastewater 2ppm, COD 1991mg / L. Then add 380g (1.0mol) 20% ammonium thiocyanate aqueous solution and 0.3g N,N-dimethyl-4-aminopyridine into the four-necked flask, and dropwis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com