A class of 3'-aminoalkoxy-luteolin derivatives and its preparation method and application
A technology of luteolin and aminoalkoxy, which is applied in the field of 3′-aminoalkoxy-luteolin derivatives and their preparation, and can solve the problems of poor water solubility, poor drugability and limited efficacy of luteolin. problem, to achieve the effect of high protection of vasoactivity, good solubility and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
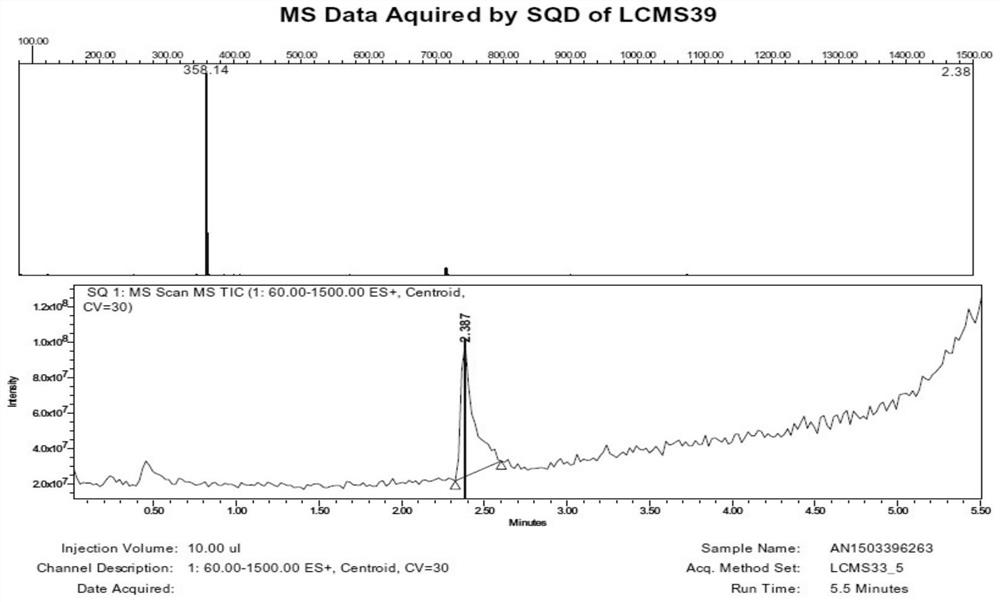
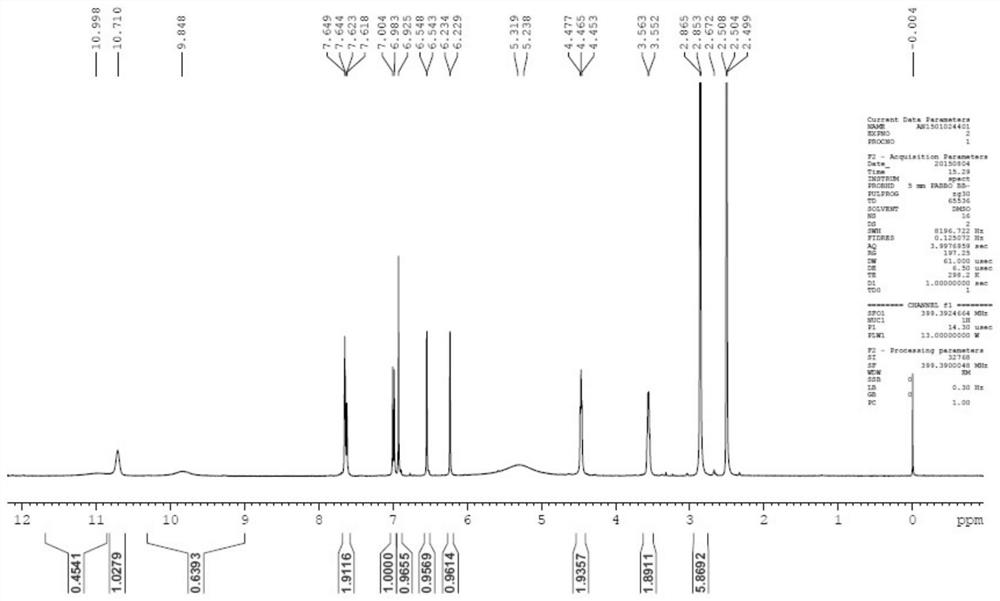
[0068] Example 1: Preparation of 3'-(dimethylamino)ethoxy-luteolin hydrochloride (1:1) (LTD2)
[0069] Under the protection of an inert gas, add 5.0g luteolin, 6.8g DIPEA and 30mL DMF into a 50mL three-necked flask, stir for 10min, and drop 4.15mL benzyl chloride in an ice-water bath. Stirring at 15-20° C. for 24-48 hours, TLC detection showed that the reaction of the raw materials was basically complete. After the reaction mixture was concentrated under vacuum, 100 mL of ethyl acetate and 200 mL of H 2 O, stirred for 30min, filtered, and the filter cake was detected as a dibenzyl substitute. The filtrate was separated into layers, the aqueous phase was discarded, and the organic phase was concentrated to obtain a crude product. The crude product was purified by silica gel column chromatography (eluent: EA / PE=1 / 5-1 / 3), and the collected fractions were spin-dried to obtain 2.0 g of khaki solid LTD-Bn2 with a yield of 24.5%. Under the protection of inert gas, 1.0g LTD-Bn2, 0....
Embodiment 2
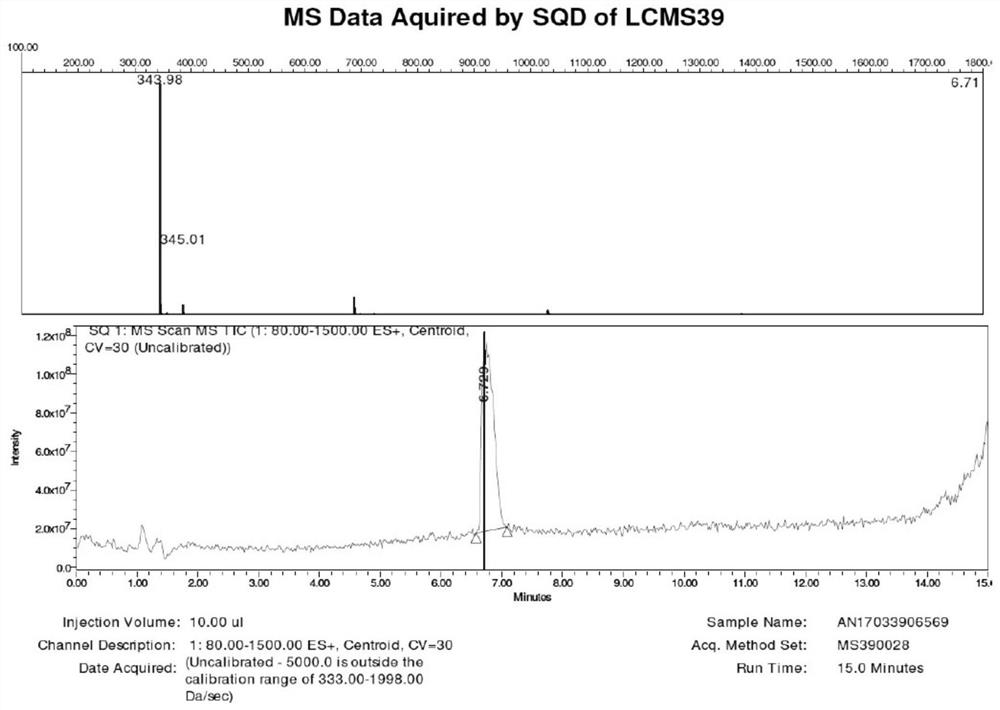
[0074] Example 2: Preparation of 3'-(monomethylamino)ethoxy-luteolin hydrochloride (1:1) (LTD7)
[0075] Under the protection of inert gas, 9.0g LTD-Bn2, 2.64g N-tert-butoxycarbonyl-N-methylaminoethanol and 4.87g PPh3 were added to a 250mL three-necked flask, stirred for 10min, and 2.92mL DEAD was added dropwise in an ice-water bath. After the dropwise addition was completed, the reaction solution was returned to room temperature, and stirring was continued for 16 h. LCMS detected that the reaction was complete. The reaction was concentrated under vacuum to give 20.0 g of crude brown product. Without further purification, it was directly used as the raw material LTD7a01 for the next reaction. Under the protection of an inert gas, 20.0 g of crude LTD7a01 was dissolved in 50 mL of dichloromethane, and 10 mL of TFA was slowly added dropwise at room temperature. After the dropwise addition, the stirring was continued for 16 h, and the reaction was monitored by LCMS to complete. ...
Embodiment 3
[0080] Example 3: Preparation of 3'-aminoethoxy-luteolin hydrochloride (1:1) (LTD8)
[0081] Under the protection of inert gas, 6.0g LTD-Bn2, 1.6mL N-(tert-butoxycarbonyl)ethanolamine and 3.38g PPh3 were added to a 250mL three-neck flask, stirred for 10min, and 1.91mL DEAD was added dropwise in an ice-water bath. After the dropwise addition was completed, the reaction solution was returned to room temperature, and stirring was continued for 16 h. LCMS detected that the reaction was complete. The reactant was concentrated under vacuum, and purified by column chromatography (DCM / MeOH=20 / 1-10 / 1) to obtain 3.3 g of light yellow crude product. Under the protection of an inert gas, 3.3 g of crude LTD8a01 was dissolved in 30 mL of dichloromethane, and 6.0 mL of TFA was slowly added dropwise at room temperature. Continue to stir for 16h after the dropwise addition. Completion of the reaction was monitored by HPLC. The reaction solution was concentrated, 30.0 mL of saturated NaHCO3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com