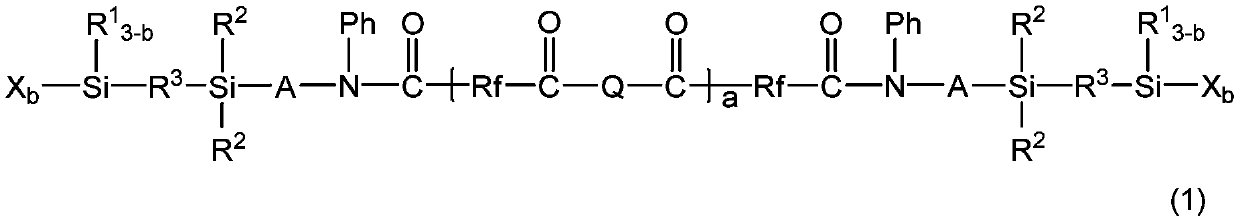
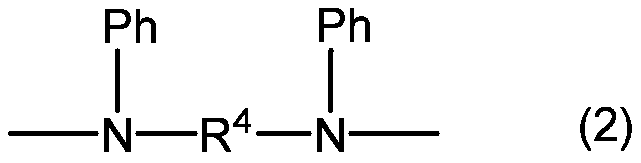
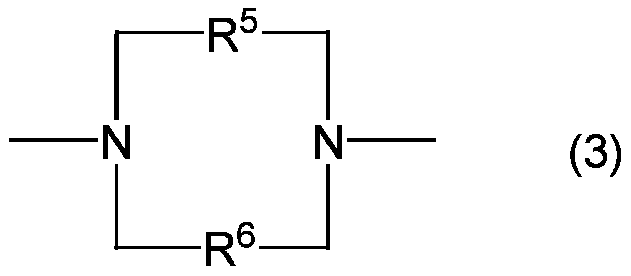
Fluorine-containing organosilicon compound, method for producing same, room temperature curable fluorine-containing rubber composition, cured product thereof, and article
A technology of organosilicon compound and fluorine-containing rubber, which is applied in the field of room temperature curing fluorine-containing rubber composition, can solve the problems of insufficient chemical resistance and durability, and achieve excellent water resistance and low moisture permeability, acid resistance and Excellent amine resistance and high fluorine content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0304] Drop into the following formula in the 300ml four-necked flask that possesses stirring rod, thermometer, serpentine condenser and dropping funnel:
[0305] 【Chemical 67】
[0306]
[0307] (In the formula, the average of p'+q' is 38.)
[0308] 189.2 g (0.030 mol, viscosity at 25°C: 430 mPa·s) of the indicated compound having acid fluoride groups at both terminals. Then, it was dripped from the dropping funnel while stirring at room temperature (25°C, the same below) by the following formula:
[0309] 【Chemical 68】
[0310]
[0311] A mixture of 15.8 g (0.072 mol) of the indicated compound and 6.5 g (0.064 mol) of triethylamine. After completion of the dropping, stirring was carried out at 60° C. for 2 hours. Next, the filtrate obtained by pressure-filtering the reaction mixture was stripped under reduced pressure at 120° C. / 3 mmHg to obtain 195.3 g of a pale yellow transparent liquid compound. This liquid compound was poured into the same flask as above, 50.0 ...
Embodiment 2
[0343] In Example 1, instead of 7.3 g of trimethoxysilane, the following formula (13) was used:
[0344] 【Chemical 74】
[0345]
[0346] Except for 7.9 g (0.050 mol) of the silane compound shown, the reaction and post-treatment were carried out in the same manner as in Example 1, and 279.3 g of a transparent oily compound tinged with pale yellow were obtained. The viscosity of the obtained oily compound was 34700 mPa·s (25°C), and the refractive index was 1.340 (25°C). the liquid compound 1 As a result of H-NMR measurement, the absorption shown below was observed.
[0347] 1 H-NMR (TMS standard)
[0348] δ=3.3~3.8ppm (m, N-C H 2 -,8H)
[0349] δ=4.1~4.2ppm (m, OC=C H 2 ,8H)
[0350] δ=6.9~7.4ppm (m, N- Ph , 20H)
[0351] In addition, this compound was hydrolyzed, and the amount of acetone desorbed was quantified. As a result, it was 0.028 mol / 100 g, which was confirmed by the following structural formula (14):
[0352] 【Chemical 75】
[0353]
[0354] (wher...
Embodiment 3
[0357] In Example 2, instead of 7.9 g of the silane compound represented by the above formula (13), the following formula (15) was used:
[0358] 【Chemical 76】
[0359]
[0360] Except for 8.1 g (0.050 mol) of the silane compound shown, the reaction and post-treatment were carried out in the same manner as in Example 2 to obtain 280.4 g of a transparent liquid compound tinged with light yellow. The obtained liquid compound had a viscosity of 35200 mPa·s (25°C) and a refractive index of 1.341 (25°C). the liquid compound 1 As a result of H-NMR measurement, the absorption shown below was observed.
[0361] 1 H-NMR (TMS standard)
[0362] δ=1.94ppm (s, SiOCC H 3 , 12H)
[0363] δ=3.3~3.8ppm (m, N-C H 2 -,8H)
[0364] δ=6.9~7.4ppm (m, N- Ph , 20H)
[0365] In addition, this compound was hydrolyzed, and the amount of acetic acid released was quantified, and the result was 0.028 mol / 100g, which was confirmed by the following structural formula (16):
[0366] 【Chemica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com