Preparation method of water-phase alkynyl amine compounds and product obtained by preparation method
A secondary amine compound and compound technology are applied in the field of preparation of aqueous alkyne amine compounds, which can solve the problems of inflammability and explosion, solvent pollution, climate change, etc., and achieve the effects of simple operation, mild conditions and wide substrate range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
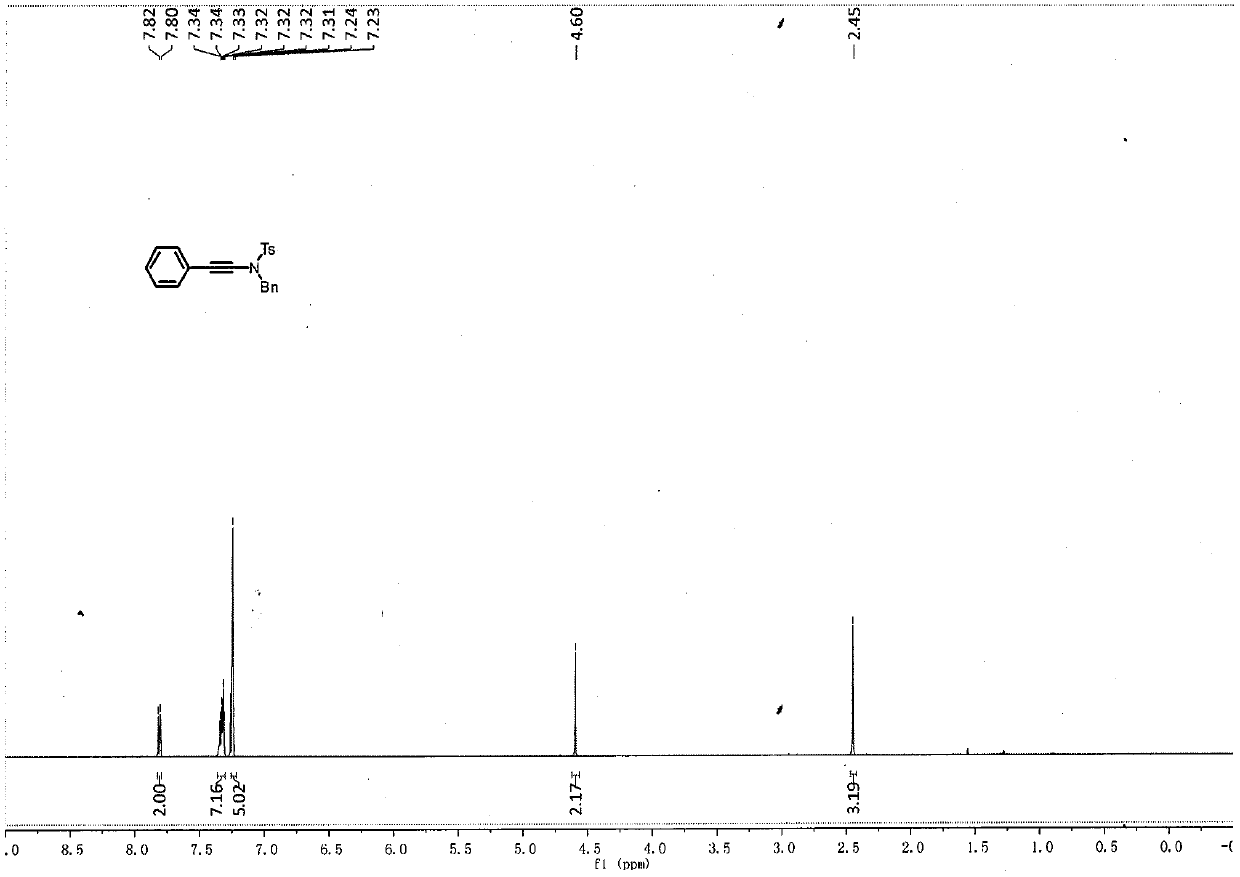
Embodiment 1
[0035]
[0036] To a pre-dried 4mL reaction flask, add benzyl-4-methylbenzenesulfonamide (53mg, 0.2mmol), copper sulfate pentahydrate (5mg, 0.02mmol), 1,10-phenanthroline ( 7.4mg, 0.04mmol), potassium carbonate (56.4mg, 0.4mmol), while adding 2wt.% surfactant APGS-550-M aqueous phase system (0.4mL), and finally adding phenylacetylene bromide (44.3mg, 0.24mmol) , after heating to 50°C, stirring was continued at 900-1000rpm for 26h.
[0037] After the reaction solution was stirred, it was extracted three times with ethyl acetate, and the organic phases extracted several times were combined into a 25mL eggplant-shaped flask, and the Heidolph rotary evaporator was used at a speed of 80-100rpm, a temperature of 38°C, and a vacuum of 0.1Mpa. , treated for 3 min, and then subjected to column chromatography using 200-mesh column chromatography silica gel, and the developer was petroleum ether: ethyl acetate = 20:1, and the target compound was isolated. (71mg, yield rate is 98%, an...
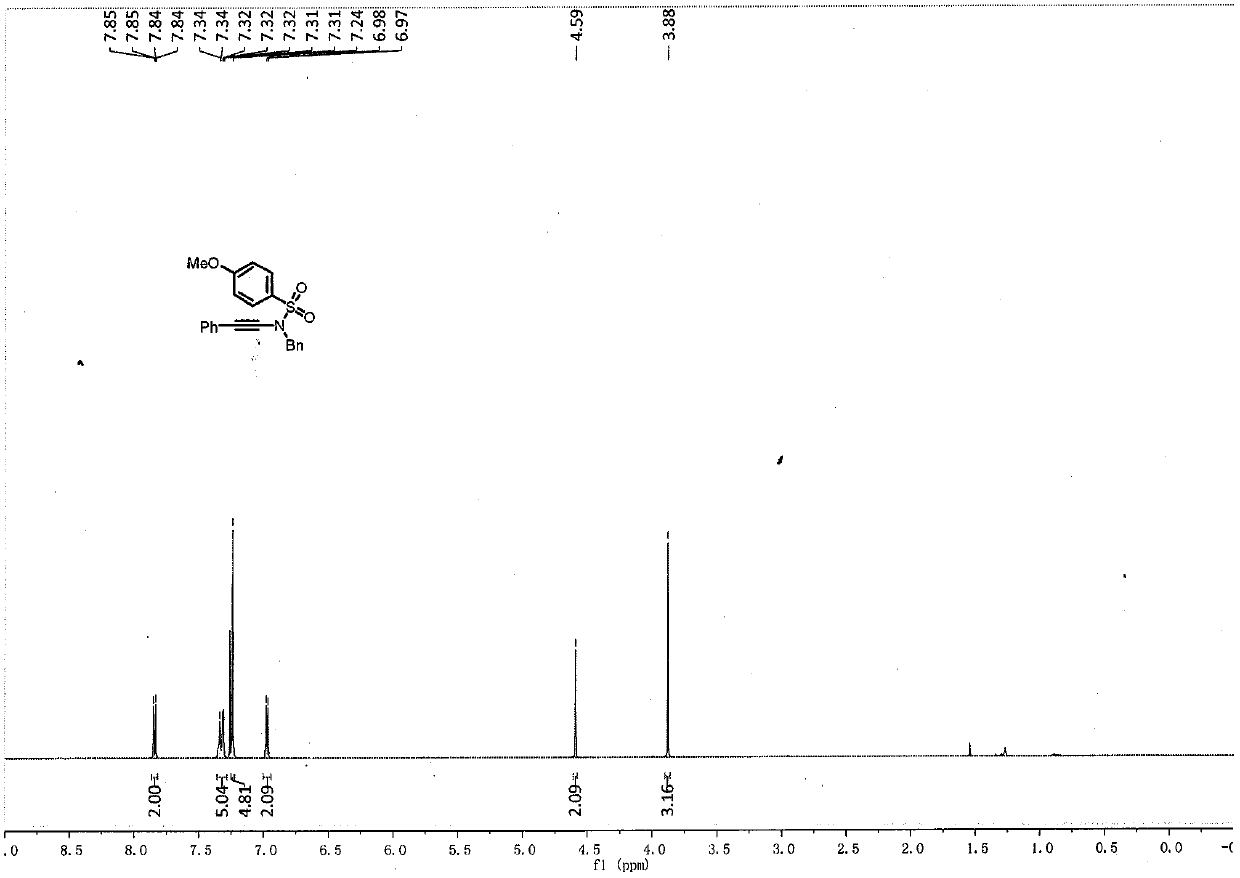
Embodiment 2
[0040]
[0041] To a pre-dried 4mL reaction flask, add N-benzyl-4-methoxybenzenesulfonamide (56.6mg, 0.2mmol), copper sulfate pentahydrate (5mg, 0.02mmol), 1,10-o- Phenanthroline (7.4mg, 0.04mmol), potassium carbonate (56.4mg, 0.4mmol), while adding 2wt.% surfactant APGS-550-M aqueous phase system (0.4mL), finally add phenylacetylene bromide (44.3mg , 0.24mmol), heated to 50°C, and continued to stir at 900-1000rpm for 26h.
[0042] After the reaction solution was stirred, it was extracted three times with ethyl acetate, and the organic phases extracted several times were combined into a 25mL eggplant-shaped flask, and the Heidolph rotary evaporator was used at a speed of 80-100rpm, a temperature of 38°C, and a vacuum of 0.1Mpa. , treated for 3 min, and then used 200 mesh column chromatography silica gel for column chromatography, and the developing solvent was petroleum ether: ethyl acetate = 15:1, and the target compound was isolated. (71 mg, the yield is 90%, and the pur...
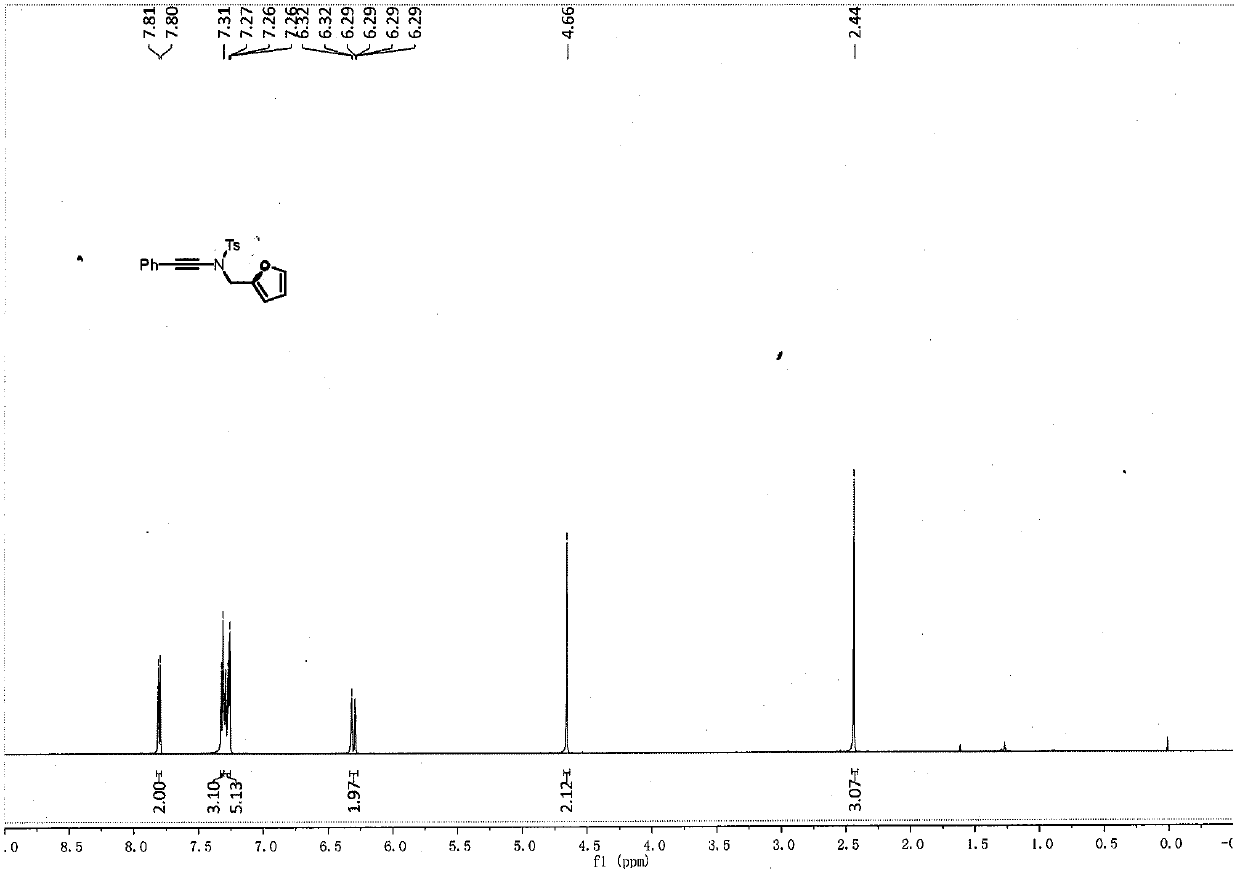
Embodiment 3
[0045]
[0046] To a pre-dried 4mL reaction flask, N-(2-furylmethyl)-4-methylbenzenesulfonamide (53mg, 0.2mmol), copper sulfate pentahydrate (5mg, 0.02mmol), 1 , 10-phenanthroline (7.4mg, 0.04mmol), potassium carbonate (56.4mg, 0.4mmol), while adding 2wt.% surfactant APGS-550-M aqueous phase system (0.4mL), finally adding phenylacetylene Bromine (44.3mg, 0.24mmol), after heating to 50°C, continued stirring at 900-1000rpm for 26h.
[0047] After the reaction solution was stirred, it was extracted three times with ethyl acetate, and the organic phases extracted several times were combined into a 25mL eggplant-shaped flask, and the Heidolph rotary evaporator was used at a speed of 80-100rpm, a temperature of 38°C, and a vacuum of 0.1Mpa. , treated for 3 min, and then subjected to column chromatography using 200-mesh column chromatography silica gel, and the developer was petroleum ether: ethyl acetate = 20:1, and the target compound was isolated. (40mg, yield rate is 81%, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com