A kind of alkynyl sulfone compound and its preparation method and application
A compound, the technology of alkynyl sulfone, which is applied in the field of alkynyl sulfone compounds and their preparation, can solve the problems of cumbersome steps and harsh conditions, and achieve the effect of simple operation, simple steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
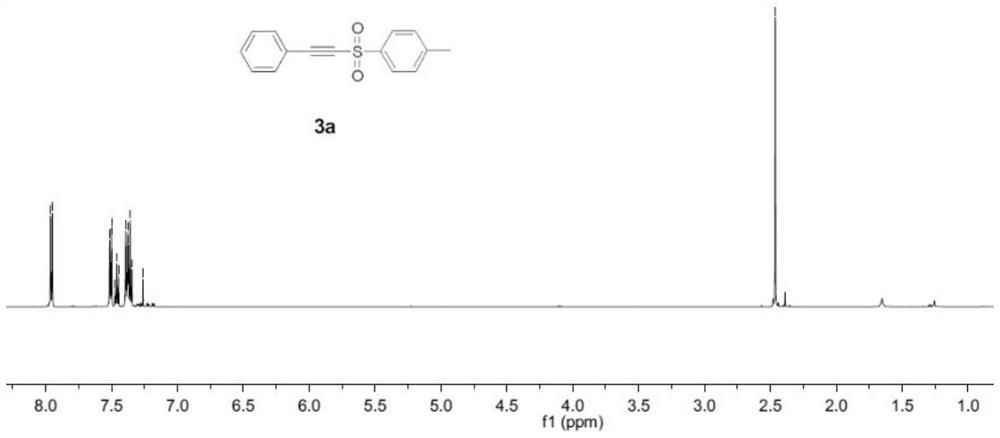
Embodiment 1
[0044] An alkynyl sulfone compound, the specific structural formula is:
[0045]
[0046] In this example, the specific synthesis steps of the alkynyl sulfone compound with the above structure are as follows:
[0047] Add phenylacetylene (0.3mmol), sodium p-toluenesulfinate (0.9mmol), KI (1.0 equivalent), H 2 O (0.1 mL) and CH 3 CN (10.0mL) was mixed to obtain a mixture. A three-necked flask was equipped with a platinum electrode (1.0cm×1.0cm×0.2mm) as an anode and a cathode. The mixture was stirred and electrolyzed at a constant current of 10mA at room temperature for 7h. After the reaction was completed, the reaction The system was transferred to a 25mL eggplant-shaped bottle, and the Heidolph rotary evaporator (80-100rpm, temperature 38°C, vacuum 0.1Mpa) was used for rotary evaporation treatment for 3min, and the residue was subjected to column chromatography with 200 mesh silica gel. Chromatography (the developer of column chromatography is sherwood oil and ethyl acet...
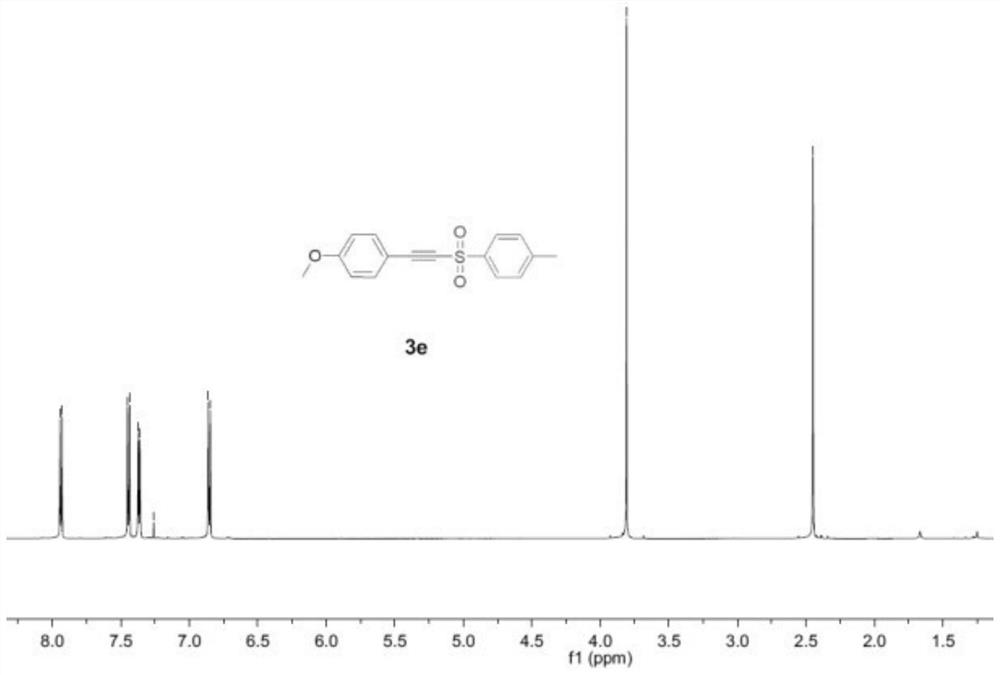
Embodiment 2
[0052] An alkynyl sulfone compound, the specific structural formula is:
[0053]
[0054] In this example, the specific synthesis steps of the alkynyl sulfone compound with the above structure are as follows:
[0055] Add p-methoxyphenylacetylene (0.3mmol), sodium p-toluenesulfinate (0.9mmol), KI (1.0 equivalent ), H 2 O (0.1 mL) and CH 3 CN (10.0mL) was mixed to obtain a mixture. A three-necked flask was equipped with a platinum electrode (1.0cm×1.0cm×0.2mm) as an anode and a cathode. The mixture was stirred and electrolyzed at a constant current of 10mA at room temperature for 7h. After the reaction was completed, the reaction The system was transferred to a 25mL eggplant-shaped bottle, and the Heidolph rotary evaporator (80-100rpm, temperature 38°C, vacuum 0.1Mpa) was used for rotary evaporation treatment for 3min, and the residue was subjected to column chromatography with 200 mesh silica gel. Chromatography (the developer of column chromatography is sherwood oil and...
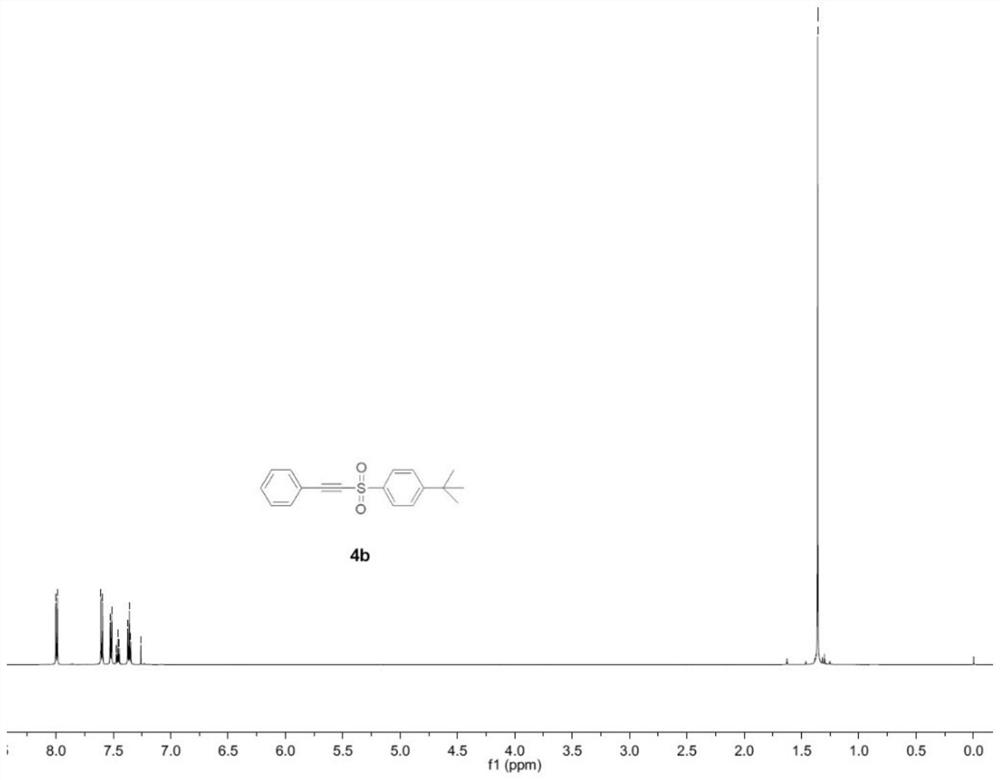
Embodiment 3
[0060] An alkynyl sulfone compound, the specific structural formula is:
[0061]
[0062] In this example, the specific synthesis steps of the alkynyl sulfone compound with the above structure are as follows:
[0063] Add phenylacetylene (0.3mmol), sodium p-tert-butylbenzenesulfinate (0.9mmol), KI (1.0 equivalent), H 2 O (0.1 mL) and CH 3 CN (10.0mL) was mixed to obtain a mixture. A three-necked flask was equipped with a platinum electrode (1.0cm×1.0cm×0.2mm) as an anode and a cathode. The mixture was stirred and electrolyzed at a constant current of 10mA at room temperature for 7h. After the reaction was completed, the reaction The system was transferred to a 25mL eggplant-shaped bottle, and the Heidolph rotary evaporator (80-100rpm, temperature 38°C, vacuum 0.1Mpa) was used for rotary evaporation treatment for 3min, and the residue was subjected to column chromatography with 200 mesh silica gel. Chromatography (the developer of column chromatography is sherwood oil and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com