A kind of preparation method of aqueous phase sulfide compound and product thereof
A compound and phase thioether technology, applied in the field of preparation of thioether compounds, can solve problems such as strong toxicity, climate change, flammability and explosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
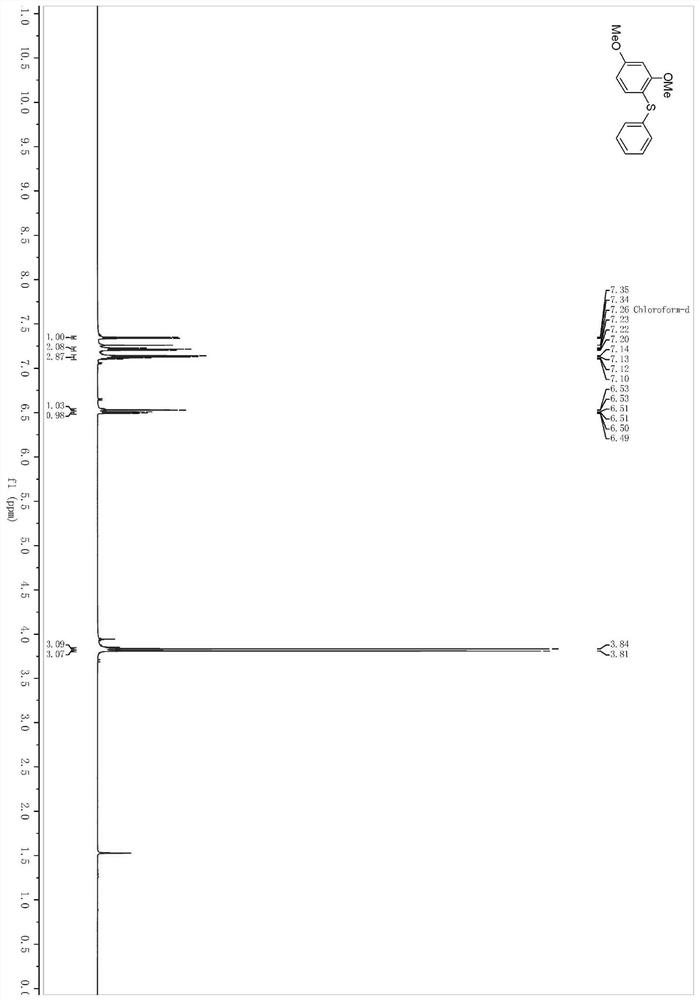
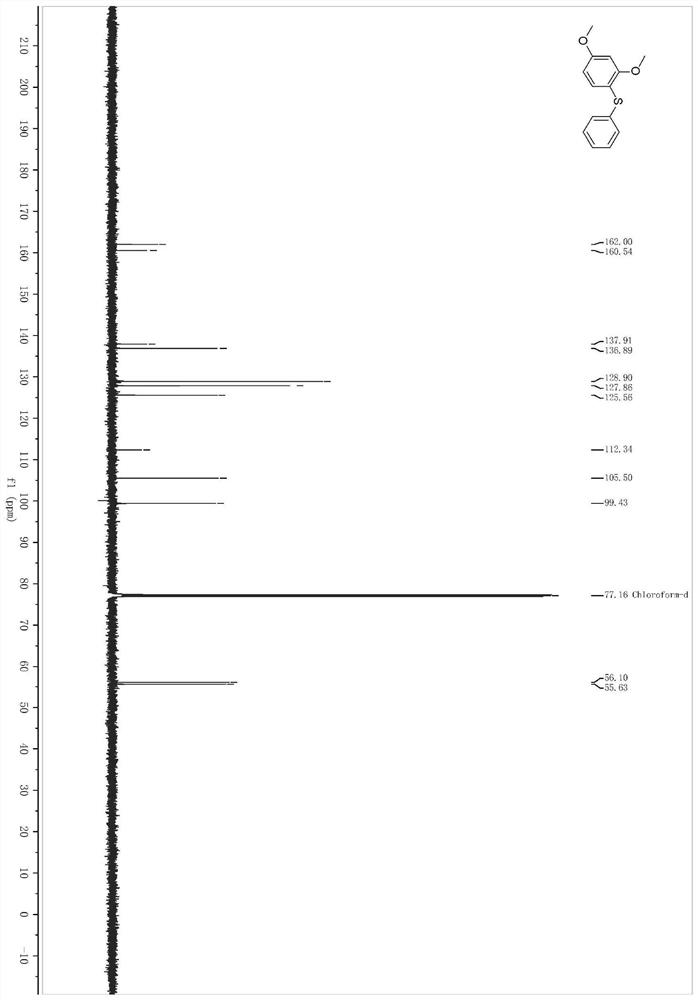
[0044] In a pre-dried 4mL reaction bottle, at room temperature, sequentially add zinc dichloride (3.28mg, 0.024mmol), 1,3-dimethoxybenzene (32.0μL, 0.24mmol), surfactant TPGS-750- M aqueous phase system (0.4mL), stir the mixture evenly, add allylphenyl sulfide (35.2μL, 0.24mmol), slowly add periodiodine compound PIFA (105.2mg, 0.24mmol) and stir at 500rpm while heating to 60°C 24h.
[0045] After the reaction solution was stirred, it was extracted three times with ethyl acetate, and the organic phases extracted several times were combined into a 25mL eggplant-shaped flask, and the Heidolph rotary evaporator was used at a speed of 80-100rpm, a temperature of 38°C, and a vacuum of 0.1Mpa. , treated for 3 min, and then subjected to column chromatography using 200-mesh column chromatography silica gel, and the developer was petroleum ether: ethyl acetate = 49:1, and the target compound 1 was isolated. (41.3 mg, the yield is 70%, and the purity analyzed by HPLC is 98%....
Embodiment 2
[0049]
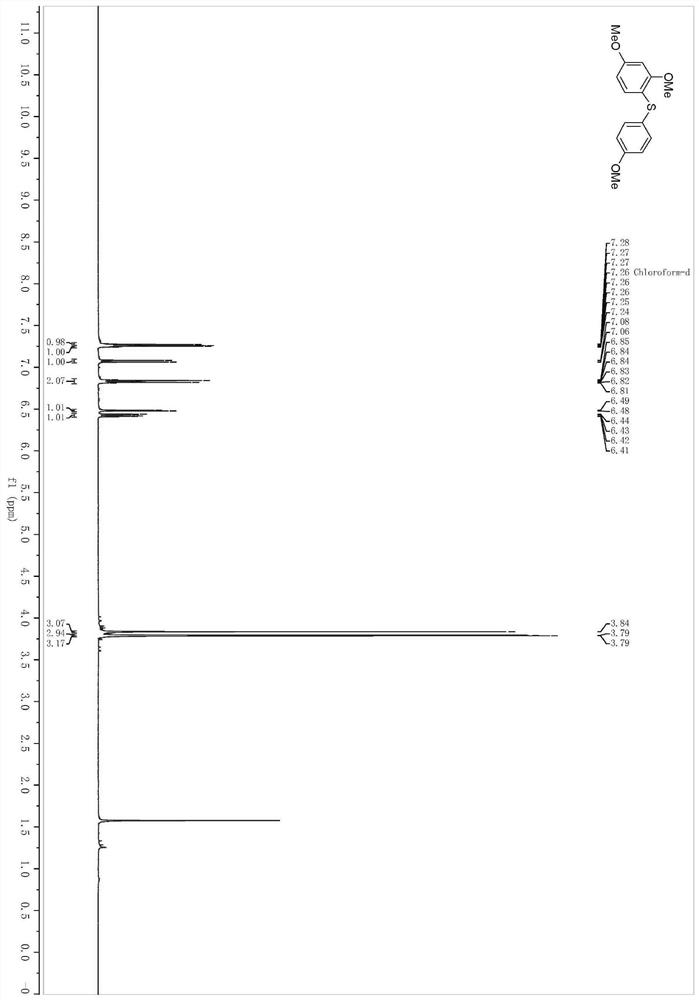
[0050] In a pre-dried 4mL reaction bottle, at room temperature, sequentially add zinc dichloride (3.28mg, 0.024mmol), 1,3-dimethoxybenzene (32.0μL, 0.24mmol), surfactant TPGS-750- M aqueous phase system (0.4mL), stir the mixture evenly, add 4-methoxyallyl phenyl sulfide (43.2mg, 0.24mmol), slowly add periodiodine compound PIFA (105.2mg, 0.24mmol) and heat to 60°C Stir at 500 rpm for 24 h at temperature.
[0051] After the reaction solution was stirred, it was extracted three times with ethyl acetate, and the organic phases extracted several times were combined into a 25mL eggplant-shaped flask, and the Heidolph rotary evaporator was used at a speed of 80-100rpm, a temperature of 38°C, and a vacuum of 0.1Mpa. , treated for 3 min, and then subjected to column chromatography using 200-mesh column chromatography silica gel, and the developer was petroleum ether: ethyl acetate = 49:1, and the target compound 2 was isolated. (34.5mg, productive rate is 52%, and the puri...
Embodiment 3
[0054]
[0055] In a pre-dried 4mL reaction bottle, at room temperature, sequentially add zinc dichloride (3.28mg, 0.024mmol), 1,3,5-trimethoxybenzene (41.2mg, 0.24mmol), surfactant TPGS-750 -M water phase system (0.4mL), the mixture was stirred evenly and added 4-methoxyallyl phenyl sulfide (43.2mg, 0.24mmol), slowly added periodiodine compound PIFA (105.2mg, 0.24mmol) and heated to 60 Stir at 500 rpm for 24 h at °C.
[0056] After the reaction solution was stirred, it was extracted three times with ethyl acetate, and the organic phases extracted several times were combined into a 25mL eggplant-shaped flask, and the Heidolph rotary evaporator was used at a speed of 80-100rpm, a temperature of 38°C, and a vacuum of 0.1Mpa. , treated for 3 min, and then subjected to column chromatography using 200-mesh column chromatography silica gel, and the developer was petroleum ether: ethyl acetate = 19:1, and the target compound 3 was isolated. (51.4 mg, the yield is 70%, and the pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com