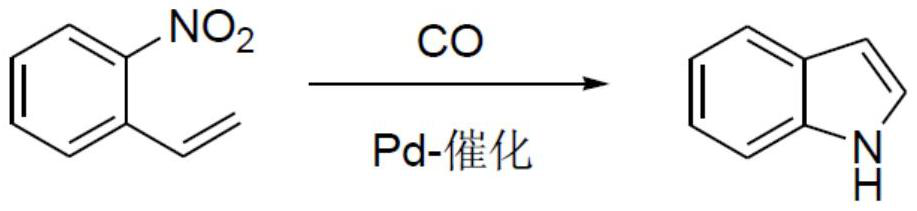
A kind of preparation method of indole compound
A compound and indole technology, applied in the field of heterocyclic compound preparation, can solve the problems of high price and complex catalyst, and achieve the effects of short reaction period, good reactivity and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the impact of different catalyst additions on synthetic effect
[0036] During the preparation process, we tried a variety of catalysts, and finally determined that silver oxide had the best catalytic effect. Using this catalyst not only improved the reactivity of raw materials, but also promoted the forward progress of the reaction; then we The addition ratio of the catalyst was also tested, see Table 1 for details.
[0037] Table 1 The influence of different catalyst additions on the synthesis effect
[0038]
[0039]
[0040]The starting material is N,N'-dimethyl(2-phenylethynyl)aniline (2mmol), silver oxide is used as catalyst, solvent is 1,2-dichloromethane (10ml), p-toluenesulfonic acid monohydrate (2mmol, i.e. 1 equivalent) exists, 40 ℃ of reaction temperature, add the catalyst of different amount according to table 1, the result shows: when catalyst amount is on the low side (referring to serial number 1,2,3 in table 1), because catalyst is ...
Embodiment 2
[0041] Embodiment 2: The influence of different atmospheric reagents on synthetic effect
[0042] In our above preparation process, the atmosphere reagent is mainly to guide the forward reaction (ie 2-ethynyl aniline derivative → indole compound). In the previous research, we initially characterized the type of atmosphere reagent as an acid reagent; In further experiments, we used the same raw materials and reaction time to conduct experiments in different systems composed of different acids, and comprehensively considered the indicators such as the time of the reaction end point, the stability of the compound, and the purity of the compound in different systems. The results showed that p-toluenesulfonic acid monohydrate (p-TSA·H 2 (2) when the guiding effect to reaction is the best, at this moment, reaction remains to carry out under middle-acid condition, and silver oxide can be fully contacted with raw material.
[0043] On the basis of the above work, we also conducted ex...
Embodiment 3
[0052] Embodiment 3: Indole (A 1 ) preparation
[0053] In a 25mL round-bottomed flask, add N,N'-dimethyl(2-phenylethynyl)aniline (442.6mg, 2mmol), silver oxide (23.1mg, 0.1mmol), p-toluenesulfonic acid monohydrate (380.4 mg, 2mmol), 1,2-dichloromethane (10mL), stirred in an oil bath at 40°C for 3 hours, spin-dried, and passed through the column with petroleum ether:ethyl acetate=50:1 to obtain 393.9mg of the target compound, light yellow Solid, yield 95%.
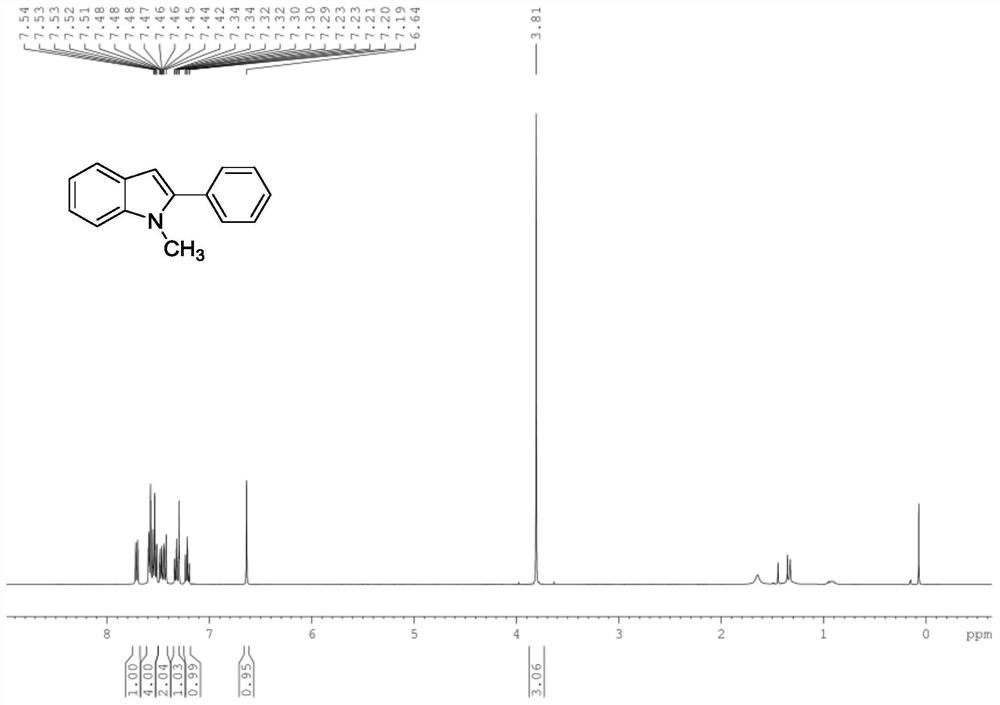
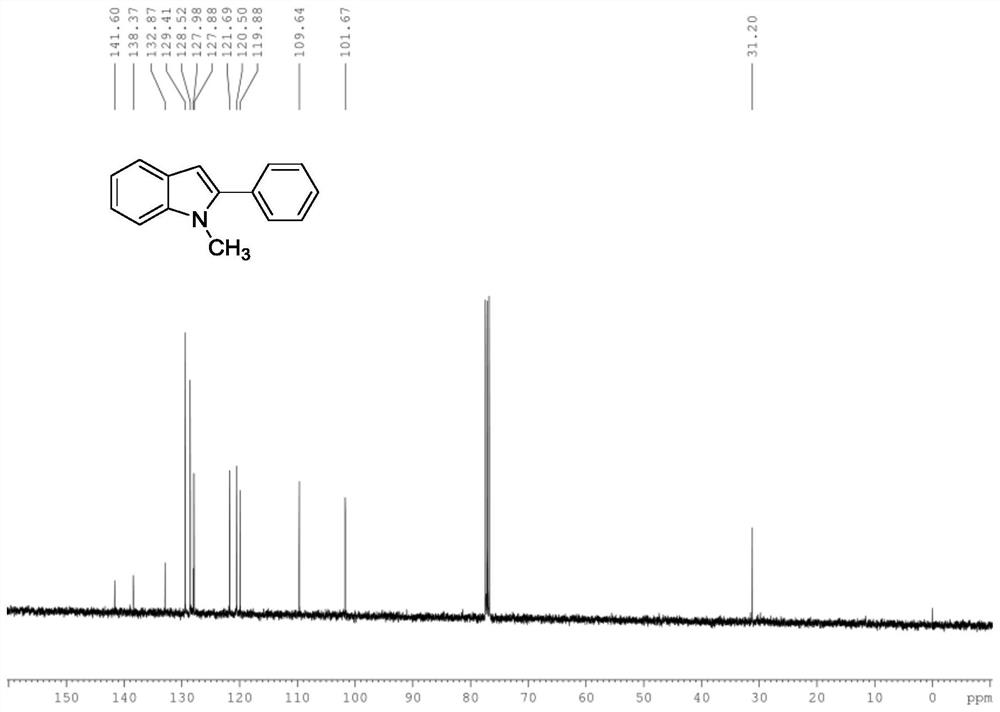
[0054] A 1 The structure is shown in formula (1), and the product structure is detected, and the results are shown in the attached Figure 1A and Figure 1B .
[0055]
[0056] 1-methyl-2-phenyl-1H-indole
[0057] 1 H NMR (400MHz, CDCl 3 )δ7.71(dt,J=8.0,1.0Hz,1H),7.61–7.50(m,4H),7.49–7.40(m,2H),7.35–7.27(m,1H),7.49–7.40(m, 1H), 6.64(s, 1H), 3.81(s, 3H);
[0058] 13 C NMR (101MHz, CDCl 3 )δ141.60, 138.38, 132.88, 129.41, 128.52, 127.99, 127.88, 121.69, 120.51, 119.89, 109.64, 101.68, 31.20.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com