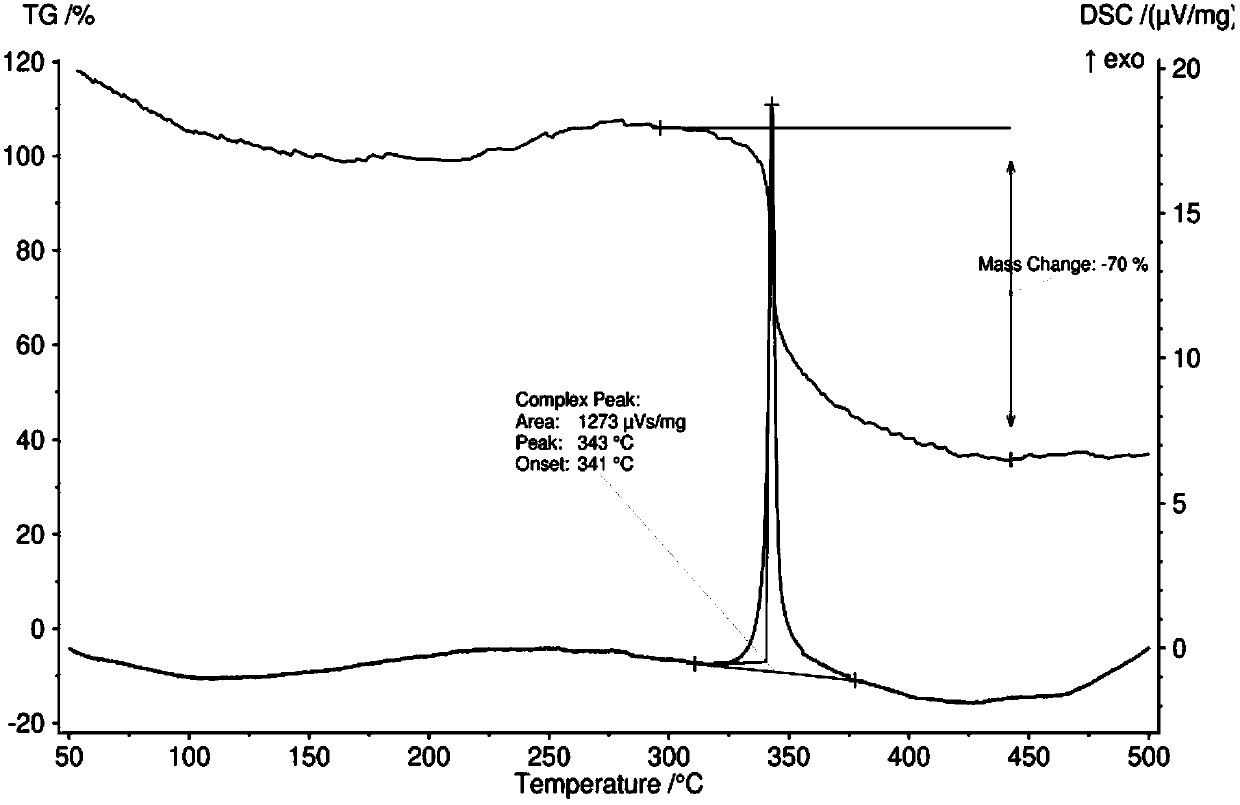
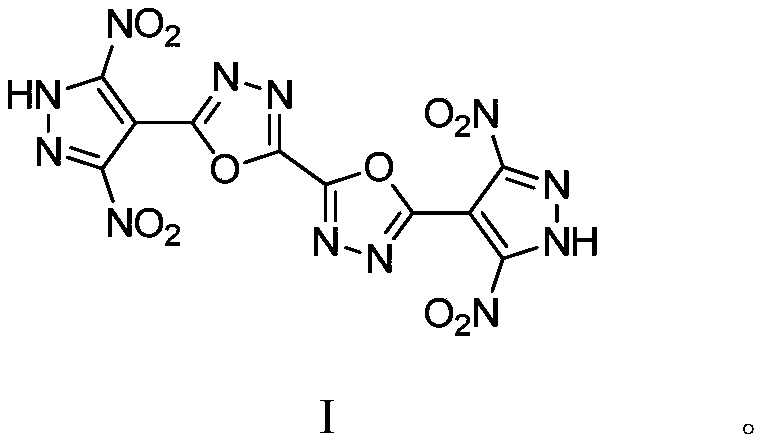
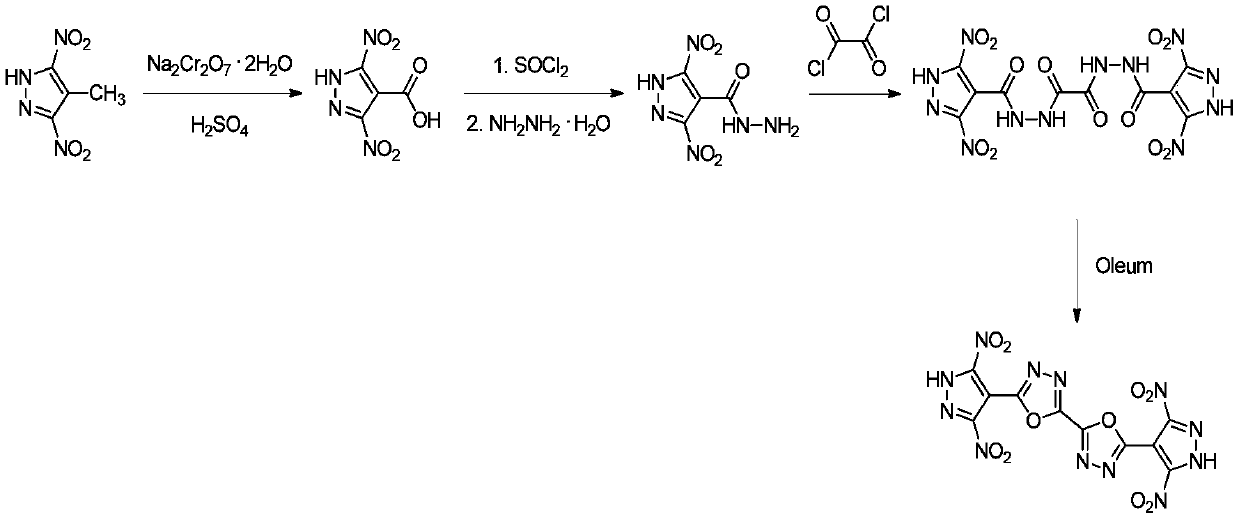
5,5'-bis(3,5-dinitropyrazolyl)-2,2'-bi(1,3,4-oxadiazole) and synthetic method thereof
A technology of dinitropyrazolyl and dinitropyrazole, which is applied in the field of 5,5'-bis-2,2'-bis compound and its synthesis, can solve the problem of low HNS density and energy, and unsatisfactory energy density Issues such as the development direction of high-energy materials for energetic materials, to achieve the effect of excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Synthesis of 4-carboxy-3,5-dinitropyrazole
[0026] Add sodium dichromate (17.88g, 0.06mol) to 4-methyl-3,5-dinitropyrazole (5.16g, 0.03mol) in concentrated sulfuric acid solution (98%, 90mL) in batches at room temperature , The reaction solution was stirred at room temperature for 12h. The reaction solution was poured into crushed ice, extracted three times with ethyl acetate, 150 mL each time, and the extract was dried and concentrated to obtain 4.08 g of a white solid, with a yield of 67.3%.
[0027] Characterization data:
[0028] 1 H NMR (400MHz, DMSO-d 6 ):δ11.67(br,1H).
[0029] 13 C NMR (100MHz, DMSO-d 6 ): δ161.2, 148.9, 108.6.
[0030] IR(ATR,cm -1 ):3166,2961,1737,1546,1488,1432,1333,1294,1257,1210,1120,1029,849,826,743.
[0031] MS(ESˉ):201.0[M-1]ˉ.
[0032] Elemental Analysis: C 4 h 2 N 4 o 6 , theoretical value C 23.77, H 1.00, N 27.73; measured value C 23.79, H 1.13, N 26.78.
[0033] The above data prove that the structure of the synth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal resistance | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com