Preparation method of improved mesenchymal stem cells and application thereof
An improved technology of mesenchymal stem cells, applied in the field of biopharmaceuticals, can solve the problems of lower homing rate of MSCs and affect the treatment effect, and achieve the effect of promoting directional migration and improving the treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0025] Sources of materials used in the examples are explained:
[0026] 6-8 week-old SPF-grade C57BL / 6 was purchased from the Institute of Model Animals, Nanjing University;
[0027] Human umbilical cord MSCs were provided by Tianjin Angsai;
[0028] Flow antibodies were purchased from BioLegend;
[0029] Dir lipid dye was purchased from Thermo Fisher Scientific, the Transwell chamber was purchased from Corning (Corning), and the human source FSTL1 recombinant protein was purchased from R&D Company;
[0030] Human MSCs-specific medium was purchased from R&D Company, and other cell culture reagents were purchased from Life Technology Company.
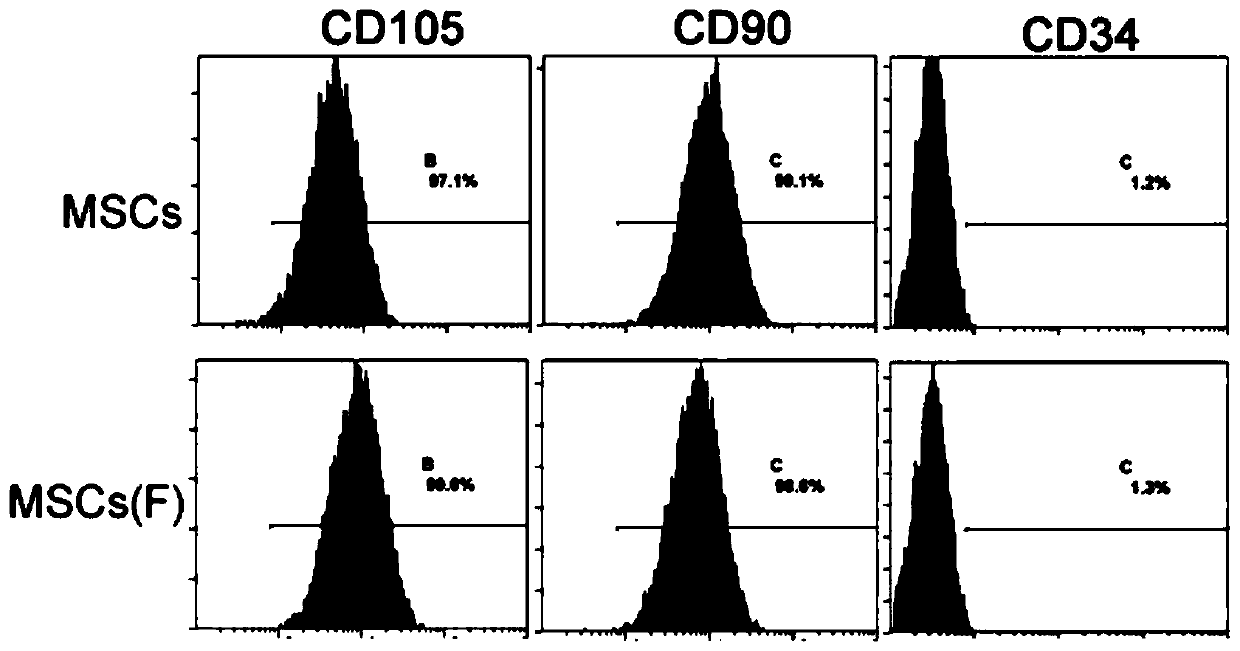
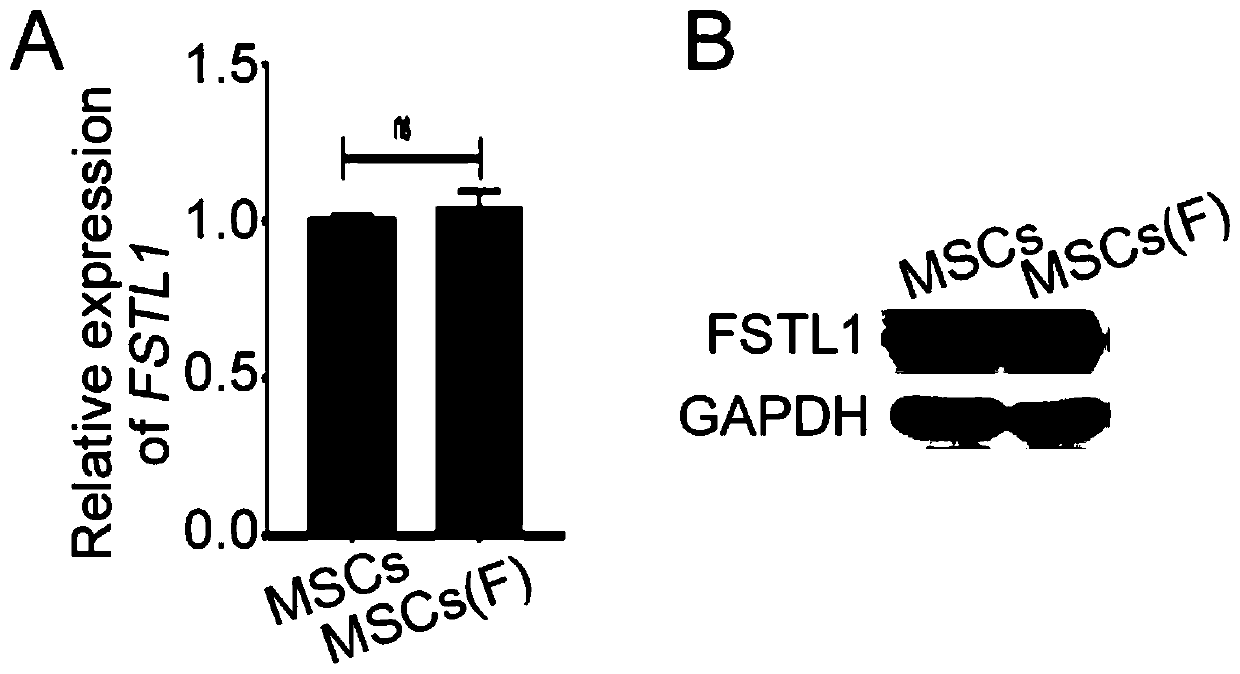
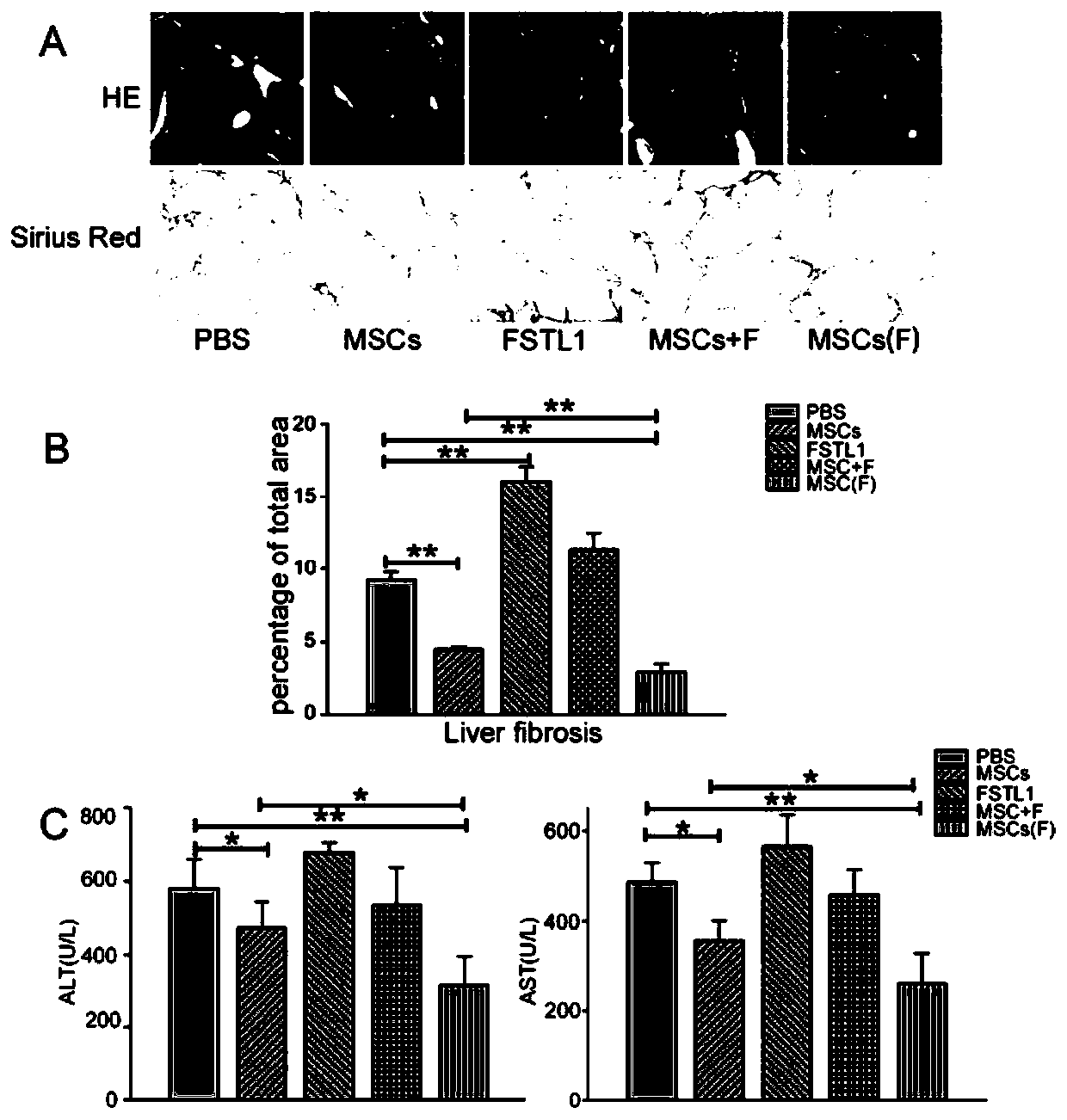
[0031] Preparation and in vitro characterization of the improved MSC of the present invention.
[0032] 37°C 5% CO 2 Human umbilical cord MSCs (containing 1‰ penicillin and 1‰ streptomycin) were used in the incubator, and when the confluence reached 90%, they were subcultured at a ratio of 1:2, and continued to be cultured until the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com