Means and methods for oral protein delivery
A fusion protein, matrix technology, applied in peptide/protein components, chemical instruments and methods, animal/human proteins, etc., can solve problems such as expensive regulatory procedures, not edible vaccines, and lengthy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Materials and methods of Example 1
[0184] Expression of VHH-IgAFc
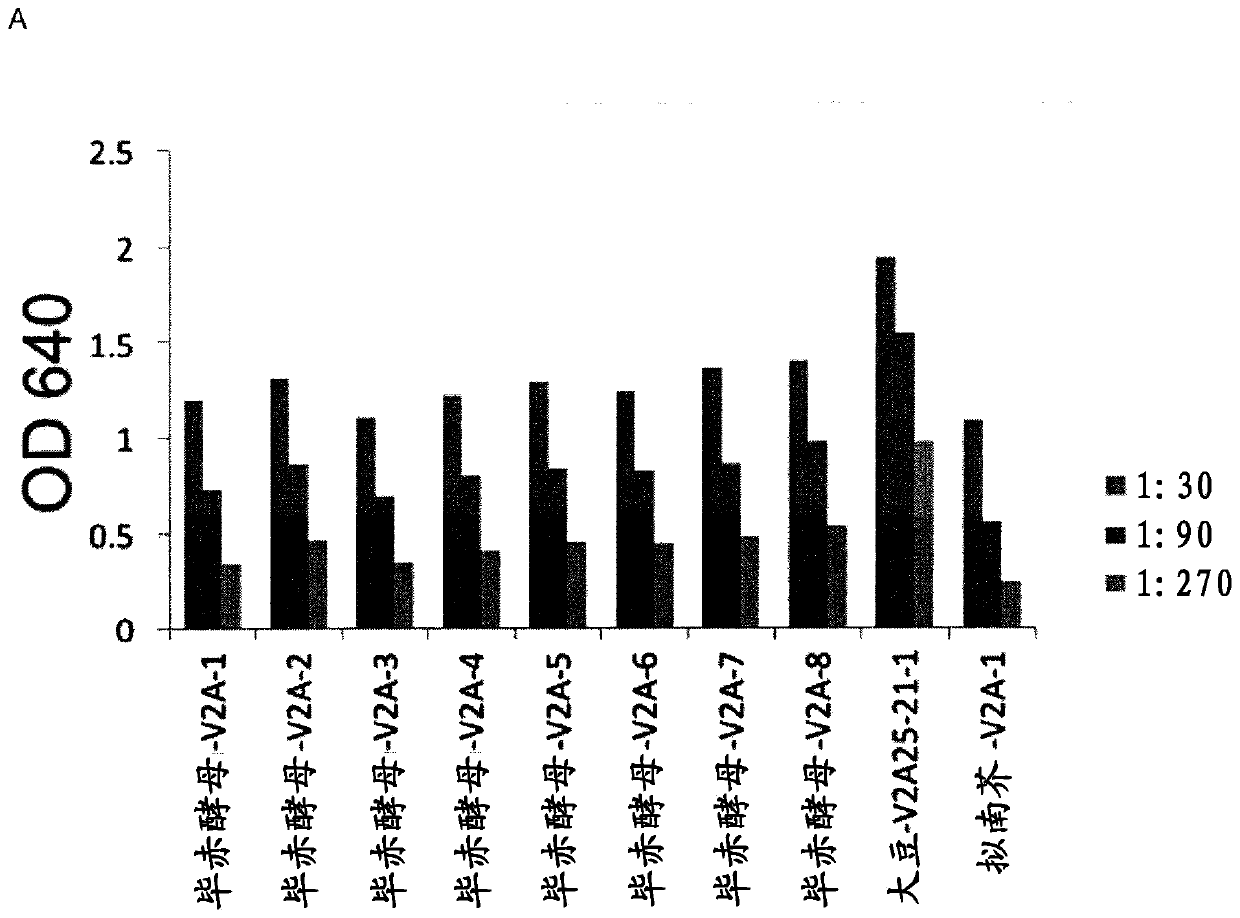
[0185] Arabidopsis: A previously published Arabidopsis line expressing monomeric V2A-IgAFc and V3A-IgAFc fusion proteins (Virdi et al. (2013) PNAS 110, 29, 11809-11814), was scaled up in the greenhouse to produce approximately 100 g of V2A- IgAFc and V3A-IgAFc produced seeds and formulated antibody-containing feeds for Arabidopsis groups in challenge experiments.
[0186] soybean : Plasmids pEV2A and pEV3A (Virdi et al. (2013) PNAS 110, 29, 11809-11814) with the VHH-IgAFc fusion gene of antibodies V2A and V3A respectively, according to Cloning instruction manual (Invitrogen), recombined into the pGW43 multisite gateway cassette (Karimi et al. (2002) Trends Plant Sci 7, 193-195), said cassette has the gene conferring phosphinothricin resistance, for use herbicide selection of transformants. The resulting expression vectors were named pMXV2A and pMXV3A, and then the expression vectors were i...
Embodiment 2
[0220] Materials and methods of Example 2
[0221] Strains, media and reagents
[0222] Escherichia coli (E. coli) MC1061 or DH5α were used for standard molecular biology procedures. For plasmid propagation, E. coli were grown in LB broth (0.5% yeast extract, 1% tryptone and 0.5% NaCl) supplemented with the appropriate antibiotic: 50 μg / mL carbenicillin ( Duchefa Biochemie), 50 μg / mL Kanamycin (Sigma) Aldrich), 50 μg / mL Hygromycin B (Duchefa Biochemie) or 50 μg / mL (Life Technologies). All PCR reactions were performed using Phusion high-fidelity polymerase (NEB). PCR reagents (such as dNTPs and primers) were ordered from Promega and IDT, respectively.
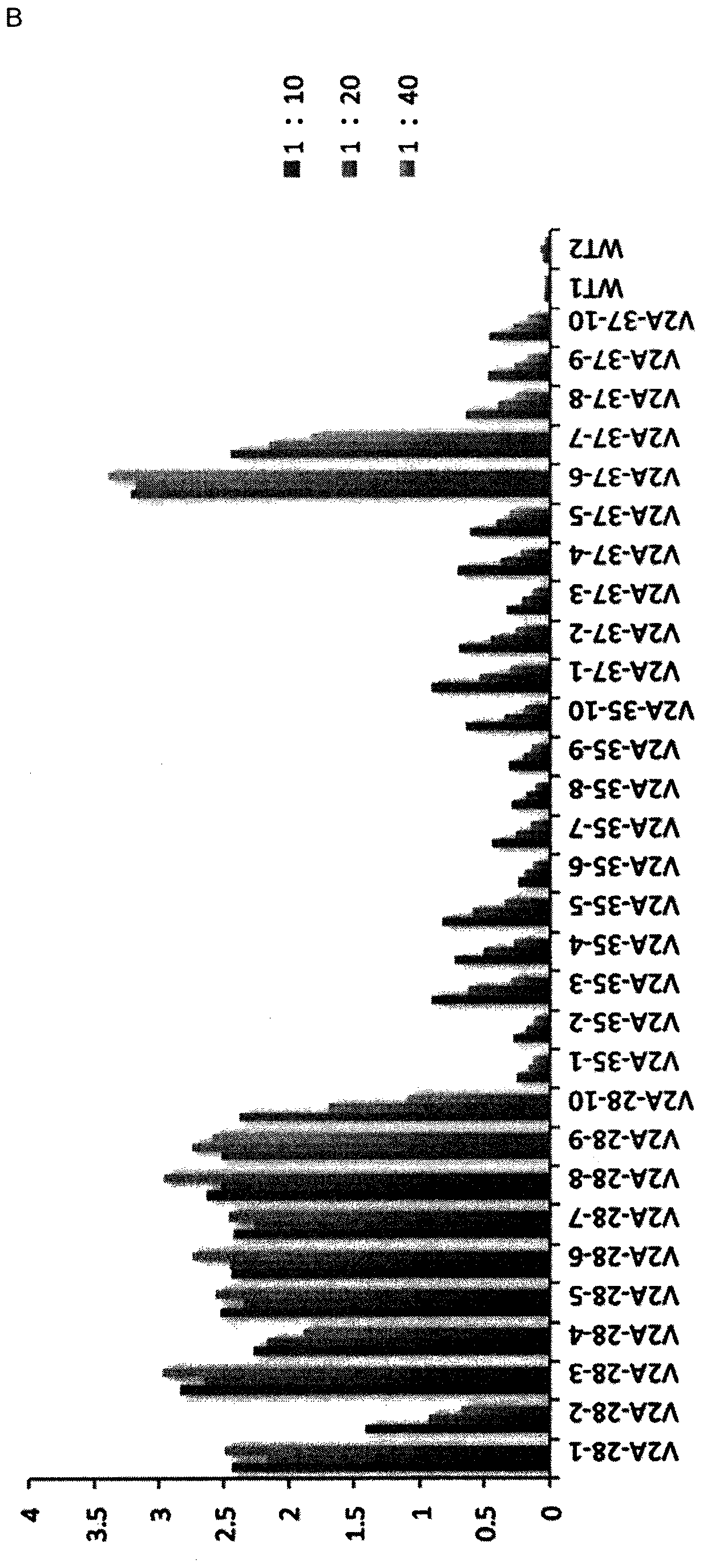
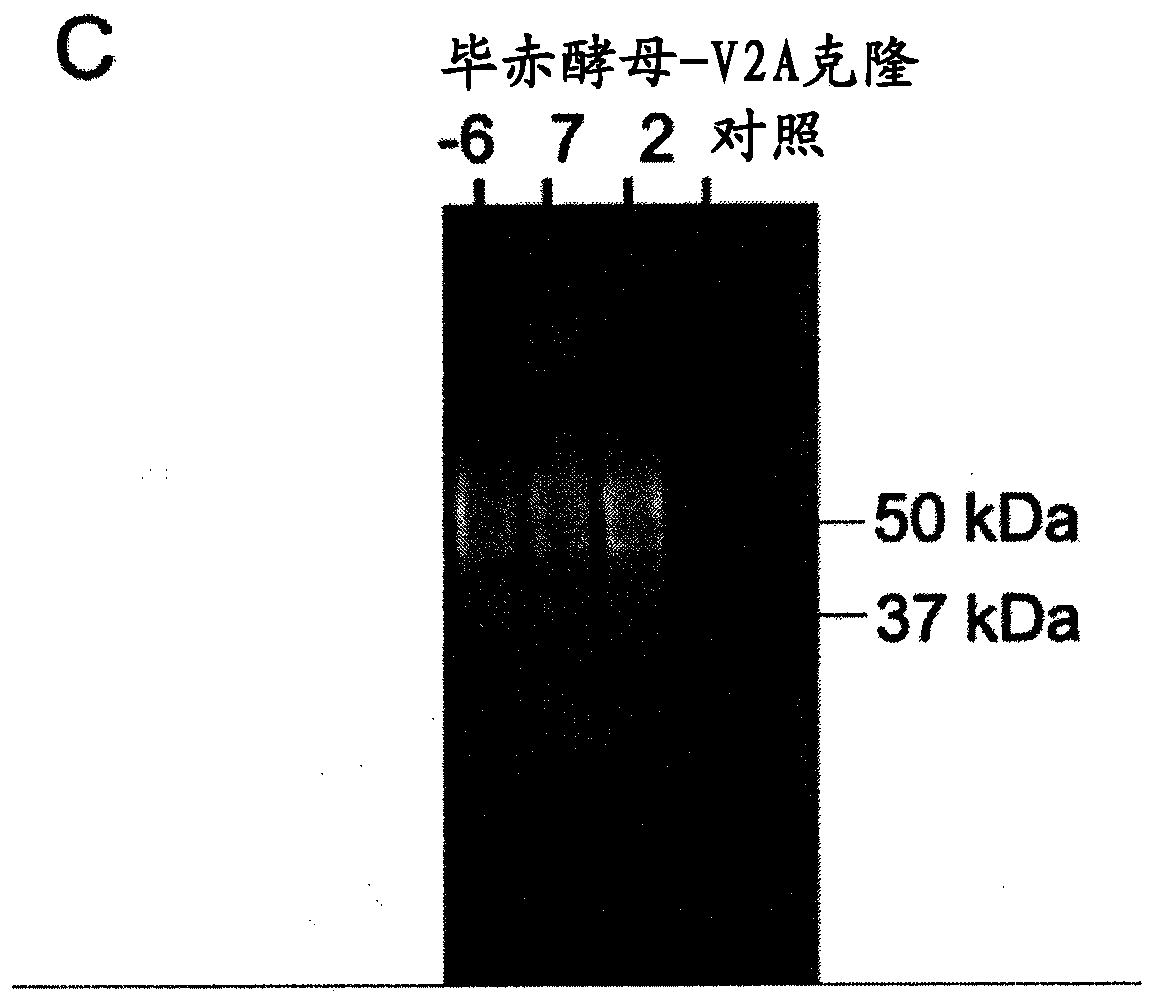
[0223] Pichia pastoris NRRL-Y 11430 strain (same name Komagataella phaffi) was provided by A. Glieder (Graz University of Technology, Austria). This strain is called wild type. Yeast culture in liquid YPD (1% yeast extract, 2% peptone, 1% D-glucose) or on solid YPD-agar (1% yeast extract, 2% peptone, 1% D-glucose, 2% ...
Embodiment 3
[0245] Materials and methods of Example 3
[0246] Feed formulation based on VHH-IgA-Fc produced by Pichia pastoris
[0247] As used in the challenge experiment of Example 1, an effective dose of anti-ETEC VHH-IgAFc is about 5 mg VHH-IgAFc, or more suitably, each piglet is fed with the dried product from a 0.5 L shake flask growth culture per day. Feed preparations. This dose consisted of two aliquots of anti-F4-ETEC VHH-IgAFcs, V2A and V3A (see Virdi et al. (2013) 110, 29, 11809-11814). To prepare similar doses for 18 piglets (three groups of six animals each) receiving Pichia-produced VHH-IgAFcs; 45 L each of Pichia cultures expressing V2A and V3A were grown in shake flasks (total 90 L ). As summarized in Table 5 below, six production batches were performed (in a five-day process, as in Example 1, growth in BMGY medium for 48 hours followed by induction in BMMY medium for 48 hours). Therefore, 15L culture batches were produced weekly. At the end of each run, the mediu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com