Salt of benzoylaminopyridine derivative and application thereof in medicines
A technology of medicine and pharmacy, applied in the field of medicine, can solve the problems of undisclosed compound crystal structure and undisclosed crystal form, and achieve the effect of convenient long-term storage and placement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
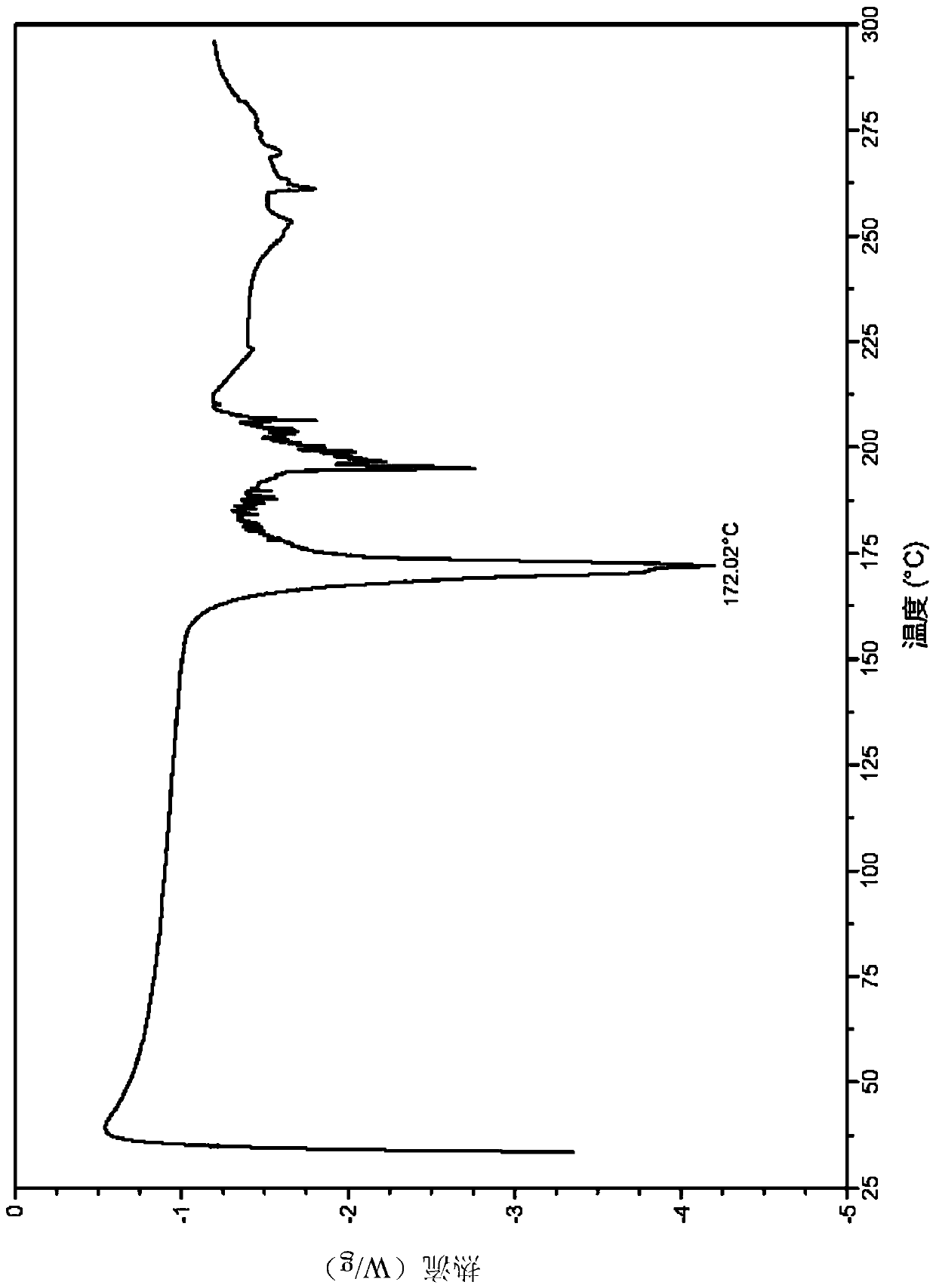
[0084] Example 1 Citrate Crystal Form I
[0085] 1. Preparation of citrate crystal form I
[0086] The compound 5-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(6,7-dihydro-5H-pyrrolo[2,1-c][1,2,4 ]triazol-3-yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide (51mg, 0.116mmol) was added to acetone (1.0mL), beaten at 60°C for 1 hour, then added lemon A solution of acid (24.2mg, 0.126mmol) in acetone (1.0mL) was reacted for 7 hours; suction filtered, and the filter cake was vacuum-dried at room temperature overnight to obtain a white solid (73.2mg, 99.6%).
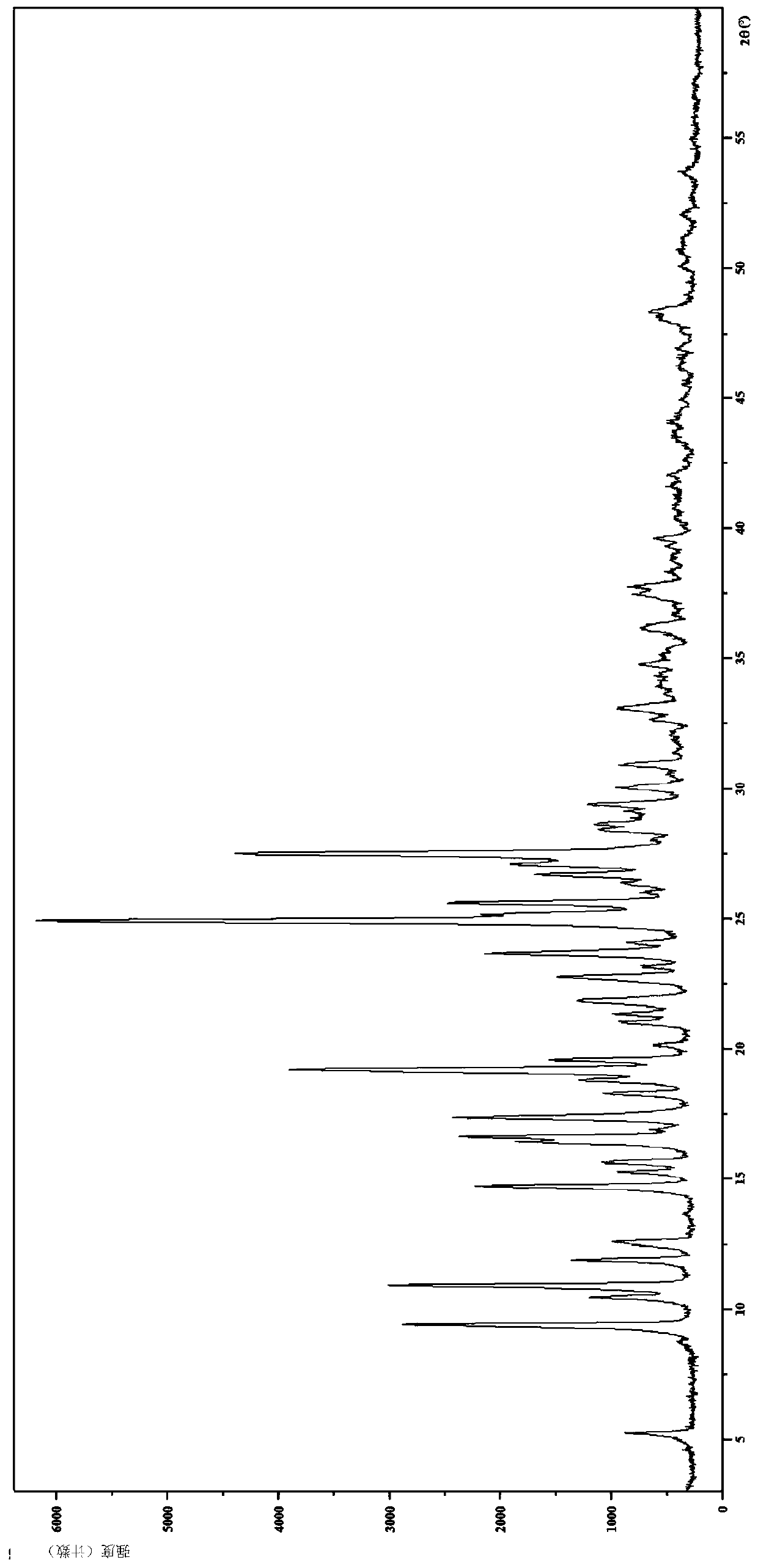
[0087] 2. Identification of citrate crystal form I
[0088] (1) Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, it has the following characteristic peaks expressed in angle 2θ: 5.20°, 8.72°, 9.35°, 10.43°, 10.86°, 11.84°, 12.50 °,13.62°,14.66°,15.24°,15.62°,16.40°,16.61°,17.35°,18.25°,18.78°,19.16°,19.54°,20.11°,20.98°,21.31°,21.80°,22.70°, 23.12°,23.63°,24.03°,24.87°,25.14°,25.56°,25....
Embodiment 2
[0090] Example 2 Citrate Amorphous
[0091] 1. Preparation of Amorphous Citrate
[0092] Compound 5-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(6,7-dihydro-5H-pyrrolo[2,1-c][1,2 ,4] Triazol-3-yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide (51.8mg, 0.117mmol) was added into dichloromethane (1.0mL) and stirred to dissolve, then added A solution of citric acid (28.5 mg, 0.148 mmol) in acetone (1.0 mL) was reacted for about 7 hours; suction filtered, and the filter cake was vacuum-dried overnight at room temperature to obtain amorphous citrate (56.8 mg, 76.5%).
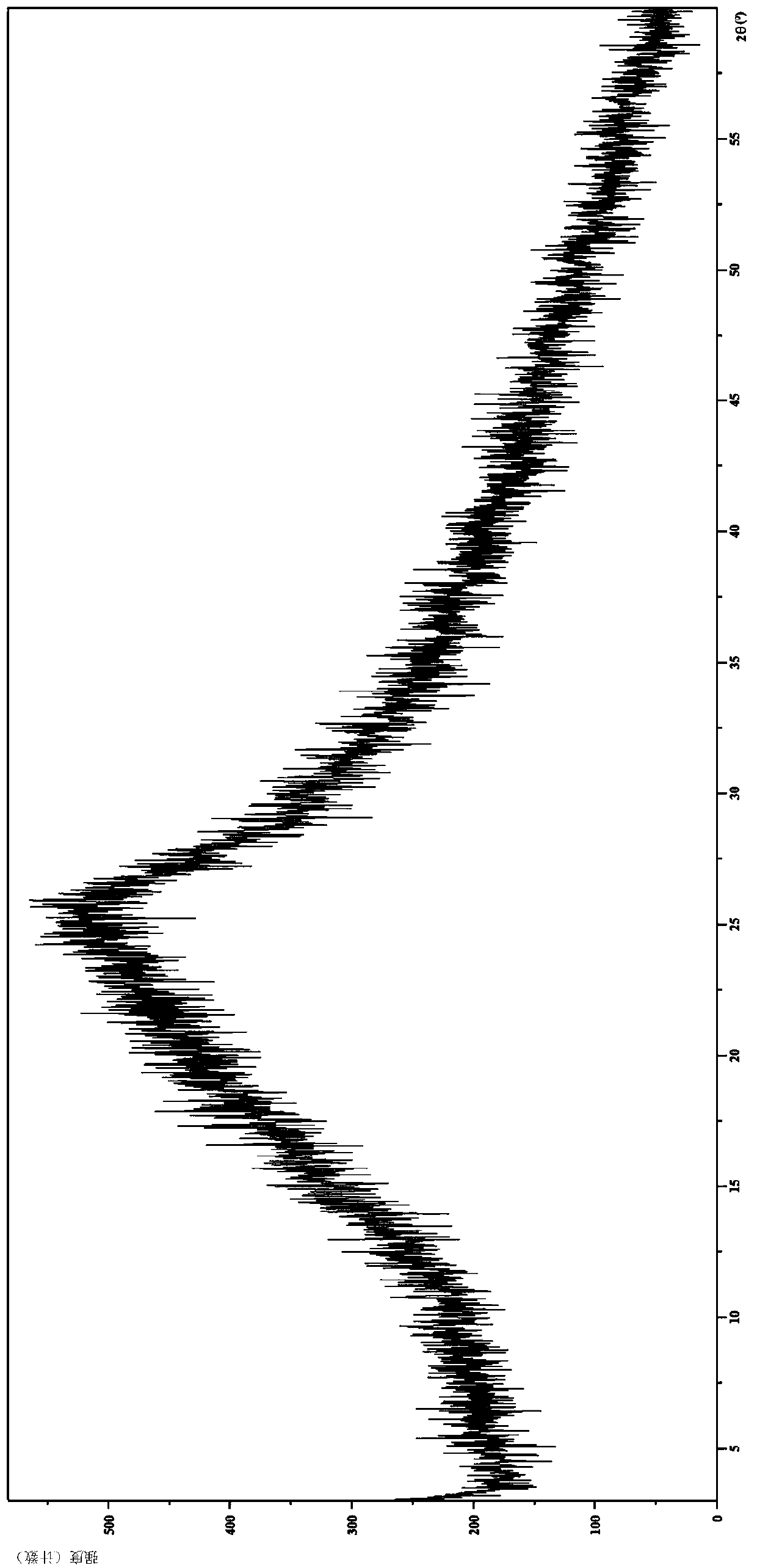
[0093] 2. Identification of Citrate Amorphous Form
[0094] Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, the experimental results are as follows figure 2 shown.
Embodiment 3
[0095] The pharmacokinetic experiment of embodiment 3 salts of the present invention
[0096] Get 8-12kg male Beagle dogs and divide them into 2 groups, 3 in each group, orally administer the capsules that test samples are housed, the dose is 5mg / kg, according to the time point 0.25,0.5,1.0,2.0,4.0,6.0,9.0 and 24h blood collection. A standard curve with an appropriate range was established according to the concentration of the sample, and the concentration of the test sample in the plasma sample was determined in the MRM mode using AB SCIEX API4000 LC-MS / MS, and quantitative analysis was performed. According to the drug concentration-time curve, the pharmacokinetic parameters were calculated by WinNonLin 6.3 software non-compartmental model method. See Table 1 for details.
[0097] Table 1 PK parameters of citrate crystal form I
[0098]
[0099] Experimental results:
[0100] As can be seen from Table 1, the citrates of the present invention all have better pharmacokin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com