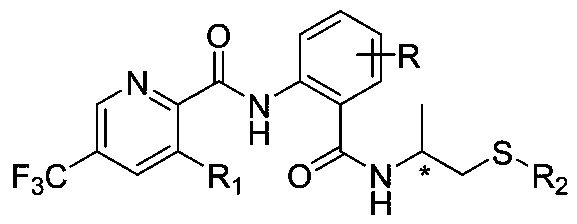
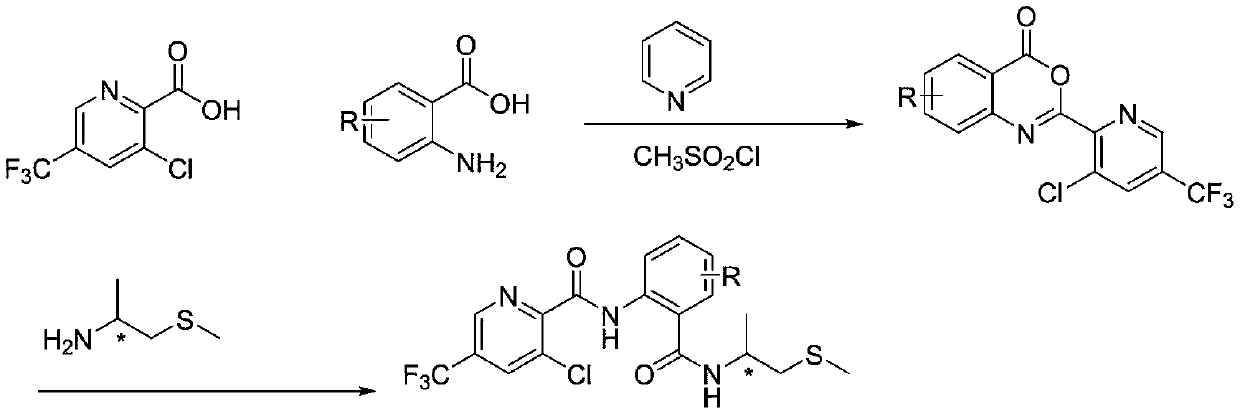
Trifluoromethylpicolinamide derivative containing chiral thioether structure and application thereof
A technology of trifluoromethylpyridinamide and sulfide, applied in the field of agrochemicals, can solve problems such as human health and ecological environment risks, achieve excellent control effects, increase selectivity, and increase the probability of interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Preparation of 1701S
[0037] first step:
[0038] 3-Chloro-5-trifluoromethylpicolinic acid (1mmol), pyridine (4mmol) and 10ml of acetonitrile were added to a 50ml three-neck flask, stirred under an ice-salt bath, and methanesulfonyl chloride ( 2mmol), after the dropwise addition, 2-amino-3-methyl-5-chlorobenzoic acid (1.3mmol) was added in one go, then slowly added pyridine (4mmol) with a constant pressure dropping funnel, 5ml of acetonitrile was added, and the dropwise addition was completed Finally, slowly add methanesulfonyl chloride (2mmol) with a constant pressure dropping funnel. After the dropwise addition is complete, remove the ice bath. After the reaction is complete, extract the reaction solution with dichloromethane and water, mix the organic layer, and use V 石油醚 :V 乙酸乙酯 =10:1 crosses column and obtains trifluoromethylpyridine oxazinone;
[0039] Step two:
[0040] Add trifluoromethylpyridoxazinone (1mmol) and 10ml of acetonitrile to start...
Embodiment 2
[0042] Embodiment 2: Preparation of 1701R
[0043] first step:
[0044] 3-Chloro-5-trifluoromethylpicolinic acid (1mmol), pyridine (4mmol) and 10ml of acetonitrile were added to a 50ml three-neck flask, stirred under an ice-salt bath, and methanesulfonyl chloride ( 2mmol), after the dropwise addition, 2-amino-3-methyl-5-chlorobenzoic acid (1.3mmol) was added in one go, then slowly added pyridine (4mmol) with a constant pressure dropping funnel, 5ml of acetonitrile was added, and the dropwise addition was completed Finally, slowly add methanesulfonyl chloride (2mmol) with a constant pressure dropping funnel. After the dropwise addition is complete, remove the ice bath. After the reaction is complete, extract the reaction solution with dichloromethane and water, mix the organic layer, and use V 石油醚 :V 乙酸乙酯 =10:1 crosses column and obtains trifluoromethylpyridine oxazinone;
[0045] Step two:
[0046] Add trifluoromethylpyridine oxazinone (1mmol) and 10ml of acetonitrile to s...
Embodiment 3
[0049] Embodiment 3: Preparation of 1702S
[0050] first step:
[0051] 3-Chloro-5-trifluoromethylpicolinic acid (1mmol), pyridine (4mmol) and 10ml of acetonitrile were added to a 50ml three-neck flask, stirred under an ice-salt bath, and methanesulfonyl chloride ( 2mmol), after the dropwise addition was completed, 2-amino-3-methylbenzoic acid (1.3mmol) was added in one go, then pyridine (4mmol) was slowly added into the constant pressure dropping funnel, 5ml of acetonitrile was added, after the dropwise addition was completed, the Slowly add methanesulfonyl chloride (2mmol) into the dropping funnel. After the dropwise addition, remove the ice bath. After the reaction is complete, extract the reaction solution with dichloromethane and water, mix the organic layer, and use V 石油醚 :V 乙酸乙酯 =10:1 crosses column and obtains trifluoromethylpyridine oxazinone;
[0052] Step two:
[0053] Add trifluoromethylpyridoxazinone (1mmol) and 10ml of acetonitrile to start stirring, and slow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com