A kind of preparation method of dihydropyridine compound
A technology of dihydropyridine and compound, applied in the field of chemical pharmacy, can solve the problems of unfavorable environmental protection, low yield of deprotection step, large waste solvent processing capacity, etc., and achieves the effects of convenient operation, low cost and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
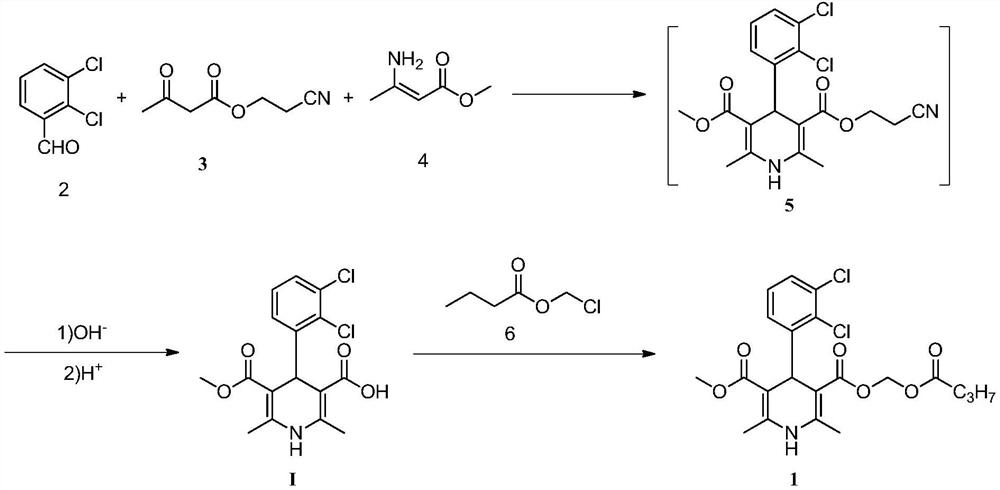
[0028] A method for preparing dihydropyridine compounds, the method uses compound II raw materials to carry out demethylation reaction in methanesulfonic acid to prepare dihydropyridine compound I;
[0029]
[0030] The reaction formula is as follows:
[0031]
[0032] Preferably, the reaction temperature is 60-80°C, and the reaction time is 3-4h.
[0033] Preferably, the reaction temperature is 80° C., and the reaction time is 3 hours.
[0034] Preferably, the weight ratio of methanesulfonic acid to compound II is 1-4:1.
[0035] Preferably, the weight ratio of methanesulfonic acid to compound II is 2:1.
[0036] Preferably, the method further includes the steps of cooling, filtering, washing and drying after the demethylation reaction.
[0037] Preferably, the temperature is lowered to 5-10° C. after the demethylation reaction.
[0038] Preferably, the washing is with cold methanesulfonic acid washing.
Embodiment 1
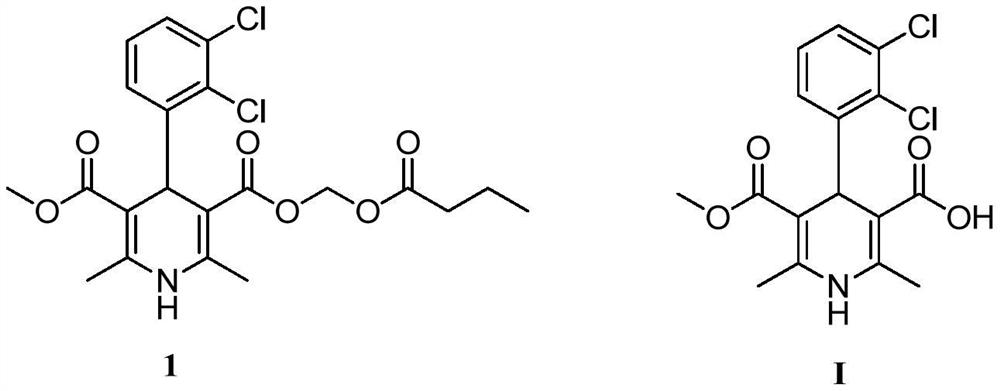
[0041] The preparation of 4-(-2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-5-methoxycarbonyl-3-pyridinecarboxylic acid (I), its reaction formula as follows:
[0042]
[0043] Methyl 4-(-2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate (II) (500g, 1.35mol) was added to methane In sulfonic acid (1000g), heat to the reaction temperature of 80°C under stirring, and stir at this temperature for 3h, then cool the reaction to 5-10°C, filter, wash the filter cake with a small amount of cold methanesulfonic acid, and dry to obtain the product ( 1) 476.2g, yield 99%.
[0044] mp.187~188℃; m / z: 356(M+H); 1 H NMR (DMSO, ppm): δ=11.57(s, 1H), 8.75(s, 1H), 7.25-7.36(m, 3H), 5.33(s, 1H), 3.51(s, 3H), 2.25(s , 3H), 2.22(s, 3H).
Embodiment 2
[0046] This example is basically the same as Example 1, except that in this example, the reaction temperature is 60° C., and the reaction time is 4 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com