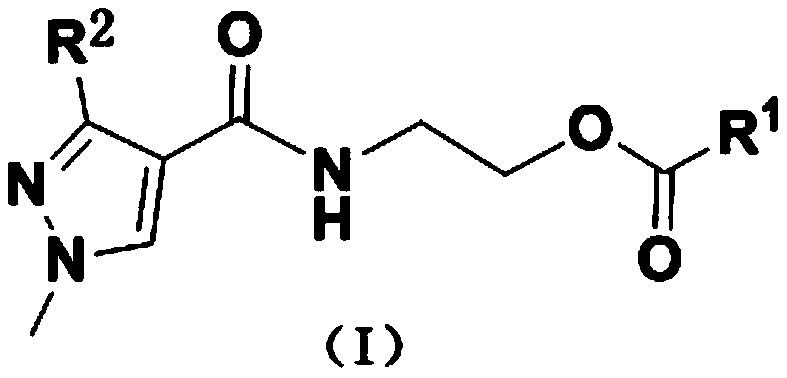
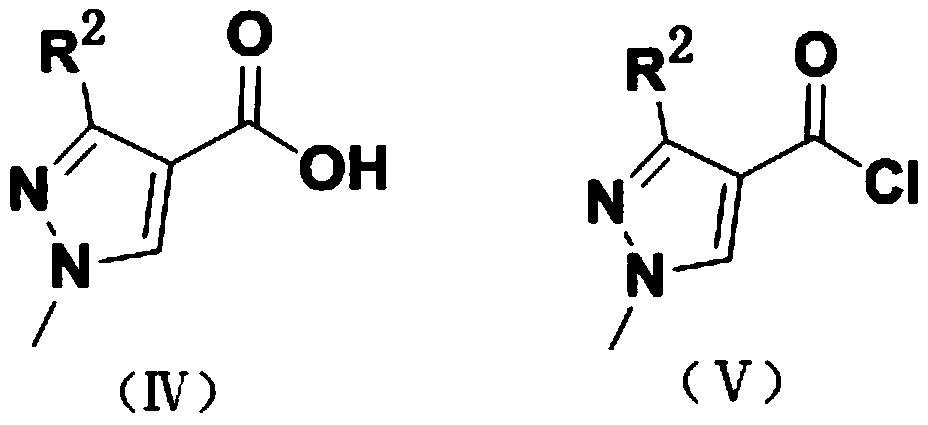
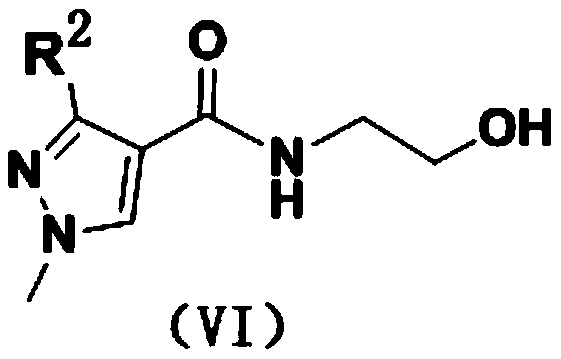
(1H-pyrazole-4-carboxamido) ethyl benzoate compound as well as preparation method and application thereof
A technology of ethyl benzoate and carboxamide group, which is applied to (1H-pyrazole-4-carboxamido) ethyl benzoate compounds and the fields of preparation and application thereof, can solve problems such as difficulty in controlling diseases and insect pests, and achieves the preparation of Simple method, convenient operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of ethyl 2-(3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamido)-2-methylbenzoate
[0032] (1) Synthesis of ethyl 4,4-difluoro-2-(methoxymethylene)-3-oxobutanoate (Ⅱ-1):
[0033] Add acetic anhydride (150.0mmol), ethyl difluoroacetoacetate (50.0mmol) and triethyl orthoformate (60.0mmol) into a 100mL reaction flask, heat up to reflux for 8 hours, stop heating, and wait for the reaction solution to cool to After room temperature, it was concentrated under reduced pressure to remove the acetic anhydride solvent, and ethyl 4,4-difluoro-2-(methoxymethylene)-3-oxobutanoate represented by formula (II-1) was obtained.
[0034] (2) Synthesis of ethyl 1-methyl-3-difluoromethyl-1H-pyrazole-4-carboxylate formula (Ⅲ-1):
[0035] Stir and mix ethyl 4,4-difluoro-2-(methoxymethylene)-3-oxobutanoate (48mmol) represented by formula (II-1) with 10mL ethanol, and then Add dropwise to the mixed solution of 8.3g of 40% methylhydrazine aqueous solution and 15mL ethan...
Embodiment 2
[0045] Example 2 Preparation of ethyl 2-(3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamido)-2,6-difluorobenzoate
[0046] The 2-methylbenzoyl chloride in the step (5) of Example 1 was replaced by 2,6-difluorobenzoyl chloride in an equivalent molar amount, and other operations were the same as in Example 1 to obtain (A2).
[0047] Ethyl 2-(3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamido)-2,6-difluorobenzoate: white solid, yield: 51.6%, mp : 102-104°C; 1 H NMR (CDCl 3 ,500MHz), δ:7.91(s,1H,py),7.49-7.44(m,1H,ph),7.36-7.41(m,2H,ph),6.81(d,J=54.5Hz,1H),6.68 (s,1H,NH),4.56-4.52(m,2H,CH 2 ),3.94(s,3H,CH 3 ), 3.81 (q, J=5.5Hz, 2H, CH 2 ); HRMS(ESI) for C 15 h 13 f 4 N 3 o 3 m / z: Calculated, 360.0966, Found, 360.0968 [M+H] + .
Embodiment 3
[0048] Example 3 Preparation of ethyl 2-(3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamido)-2,6-dichlorobenzoate
[0049] The 2-methylbenzoyl chloride in the step (5) of Example 1 was replaced by 2,6-dichlorobenzoyl chloride in an equivalent molar amount, and other operations were the same as in Example 1 to obtain (A3).
[0050] Ethyl 2-(3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamido)-2,6-dichlorobenzoate: white solid, yield: 47.3%, mp : 92-94°C; 1 H NMR (CDCl 3 ,500MHz), δ:7.84(s,1H,py),7.24(d,J=4.0Hz,2H,ph),7.19-7.16(m,1H,ph),7.04-6.81(m,2H,CHF 2 ,NH),4.46(t,J=5.5Hz,2H,CH 2 ),3.81(s,3H,CH 3 ), 3.67 (q, J=5.5Hz, 2H, CH 2 ); HRMS(ESI) for C 15 h 13 Cl 2 f 2 N 3 o 3 m / z: Calculated, 392.0375, Found, 392.0380 [M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com