Piribedil sustained-release tablet and preparation method thereof
A technology for piribedil and sustained-release tablets, which can be applied to pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve problems such as splitting phenomenon, limited types of excipients, and poor compressibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
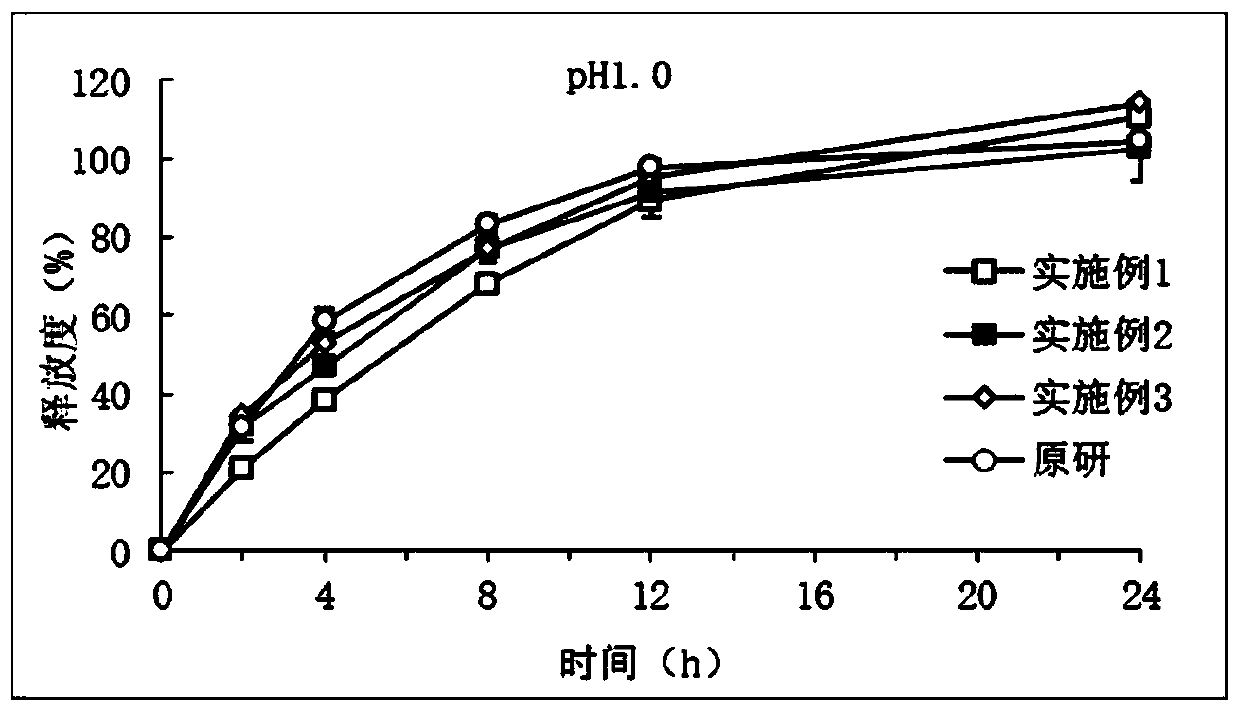
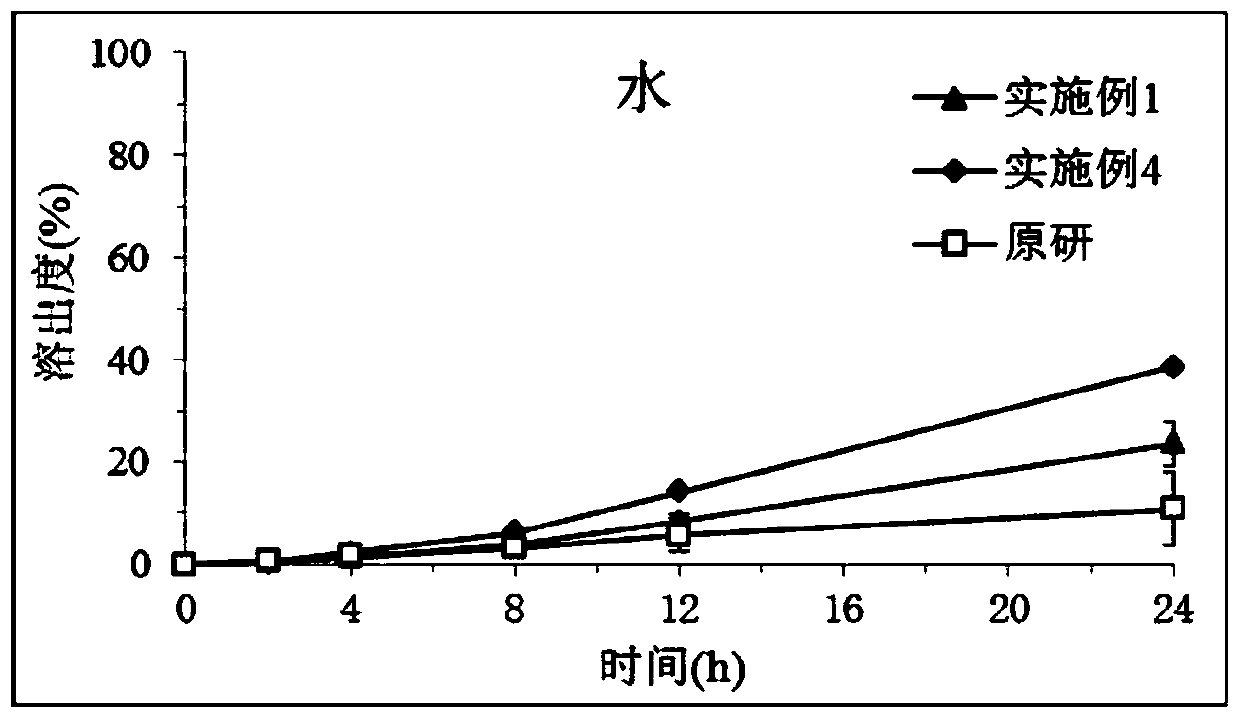
Image
Examples
Embodiment 1
[0023] Embodiment 1: Preparation of piribedil sustained-release tablets
[0024] Weigh 0.4g piribedil (small particle size < 200µm), 4g hypromellose K15M, 1g lactose, 3g acrylic resin RSPO, mix uniformly according to the method of equal addition, and use 0.5g PVPK30 in 10ml ethanol solution as Adhesive soft material, granulated with 30 mesh sieve, dried at 60°C for 20 minutes, granulated with 30 mesh sieve, added 0.6g piribedil (large particle size 200~600µm), 0.2g magnesium stearate and 0.3g Silica, mixed, compressed into tablets.
Embodiment 2
[0025] Embodiment 2: Preparation of piribedil sustained-release tablets
[0026] Weigh 0.5g piribedil (small particle size < 200μm), 1g hydroxypropyl methylcellulose K4M, 3g microcrystalline cellulose, 2g acrylic resin RSPO, mix uniformly according to the method of equal addition, and use 0.5g PVPK30 10mL ethanol solution is the soft material made of binder, granulate with 30 mesh sieve, dry at 60°C for 20min, after granulation with 30 mesh sieve, add 2.5g piribedil (large particle size 200~600μm), 0.2g stearic acid Magnesium and 0.3g of silicon dioxide, mixed, pressed into tablets.
Embodiment 3
[0027] Embodiment 3: Preparation of piribedil sustained-release tablets
[0028] Weigh 1g of piribedil (small particle size < 200µm), 6g of hydroxypropyl methylcellulose K100M, 4g of a mixture of pregelatinized starch and mannitol, and 4.7g of acrylic resin RSPO, and mix them uniformly according to the method of equal addition. Use 20mL of ethanol solution containing 0.2g PVPK30 as a binder to make soft materials, granulate with a 30-mesh sieve, dry at 60°C for 20min, and granulate with a 30-mesh sieve, add 4g of piribedil (large particle size 200-600µm), 0.05 g magnesium stearate and 0.05 g silicon dioxide, mix well, and compress into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com