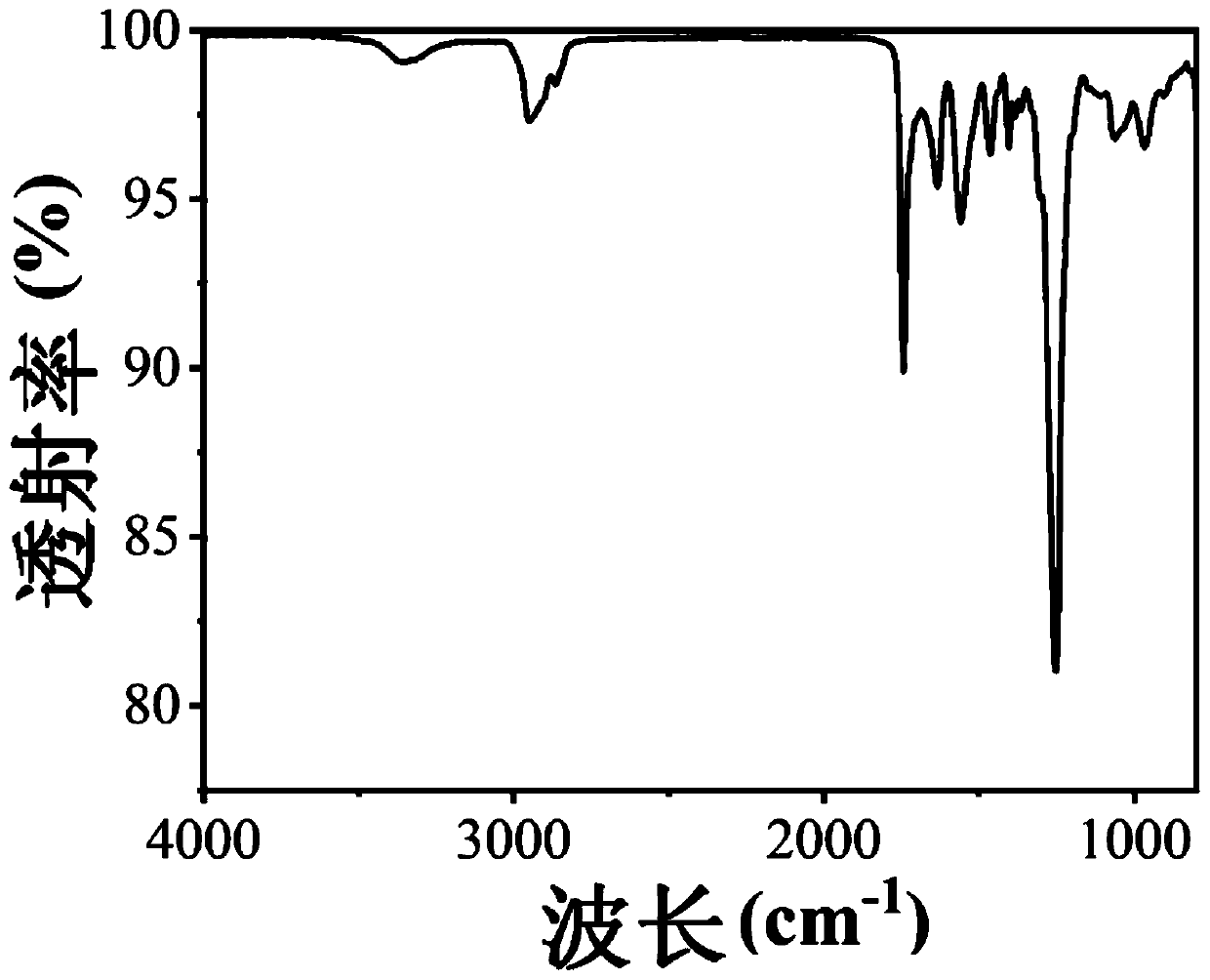
Polyurethane urea, preparation method thereof and super-tough polyurethane urea based thereon
A technology based on polyurethane urea and polyester, which is applied in the fields of biomedical polymer materials and supramolecular chemistry, can solve problems such as insufficient mechanical properties and insufficient cell affinity, and achieve improved mechanical properties, good biocompatibility and Effect of improving degradability and tensile strength at break
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
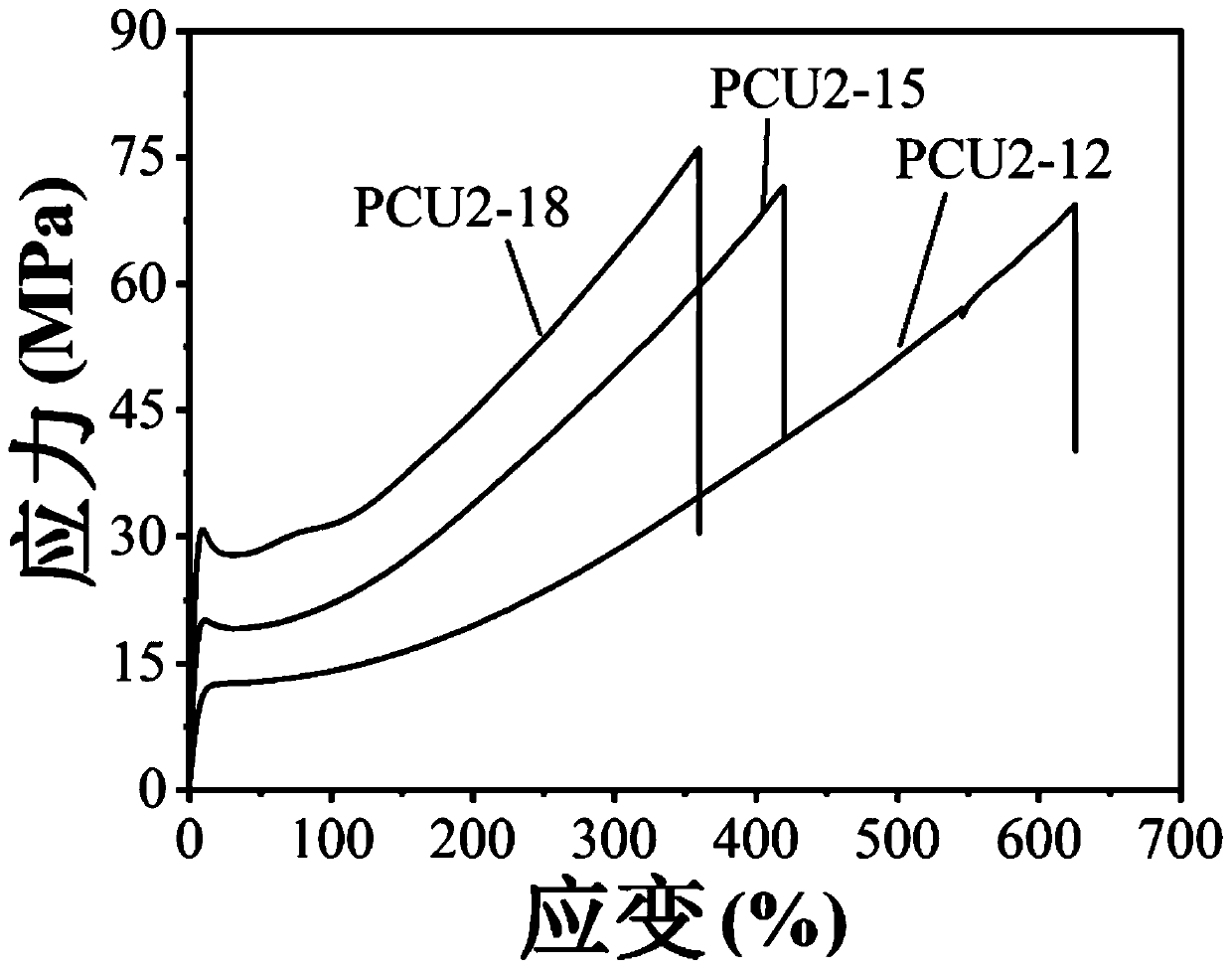
[0057] This embodiment provides three kinds of polyurethane urea and the super-tough polyurethane urea synthesis method based on the above three kinds of polyurethane urea, the specific steps are as follows:
[0058] Dissolve polycarbonate diol (PCDL) with a molecular weight of 2000 g / mol, isophorone diisocyanate (IPDI), and dimethylol butyric acid (DMBA) in DMAC solution, and then add 10 μL of catalyst dibutyltin dilaurate , Reaction at 70°C for 4h. Control the molar ratio of reactant, namely PCDL:DMBA:IPDI=2mol:1mol:nmol. In this embodiment, the values of n are 12, 15 and 18 respectively. After the reaction was completed, the temperature was lowered to 50°C, and 2(n-3)mol H 2 O continued to react for 48h. After the reaction, the reaction solution was slowly poured into ether to obtain a white flocculent solid. The product was washed with ether, dried, dissolved in DMAC, poured into a polytetrafluoroethylene mold, and dried at 70°C to obtain a polyurethaneurea film afte...
Embodiment 2
[0065] This embodiment provides a polyurethane urea and a method for synthesizing a super-tough polyurethane urea based on the above-mentioned polyurethane urea. The specific synthesis steps are as follows:
[0066] PLA with a molecular weight of 2000 g / mol and PCDL with a molecular weight of 2000 g / mol were mixed at a molar ratio of 1:1 and dissolved in DMAC solution, adding isophorone diisocyanate and dimethylol butyric acid, and then adding 10 μL of catalyst two Dibutyltin laurate, react at 70°C for 4h. Control the molar ratio of reactant, namely PLA+PCDL:DMBA:IPDI=2mol:1mol:12mol. Cool down to 50°C, add 18mol H 2 O continued to react for 60h. After the reaction, the reaction solution was slowly poured into ether to obtain a white flocculent solid. Wash the product with ether and dry it, dissolve it in DMAC, pour it into a polytetrafluoroethylene mold, and wait for the solvent to evaporate and dry at 70°C to obtain a polyurethane urea film, which is named PCPLAU 2-12.
...
Embodiment 3
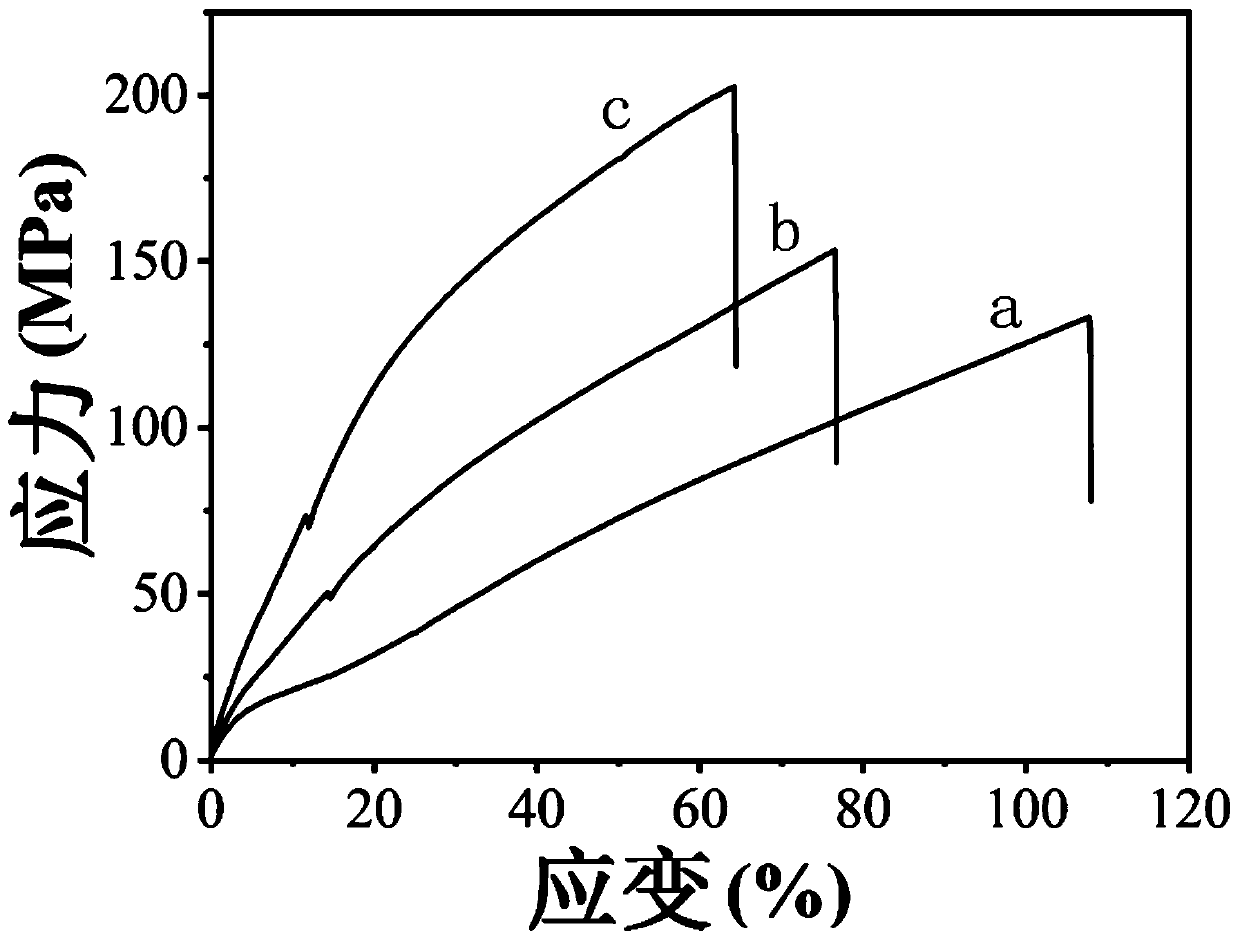
[0072] This embodiment provides a polyurethane urea with high strength, and the specific preparation steps are as follows:
[0073] Polycarbonate diol (PCDL) with a molecular weight of 2000 g / mol, HMDI, and dimethylolbutyric acid (DMBA) were dissolved in DMAC solution, and then 10 μL of catalyst dibutyltin dilaurate was added, and reacted at 70°C for 4 h. The molar ratio of the reactants is controlled, namely PCDL:DMBA:HMDI=2mol:1mol:15mol. Cool down to 50°C, add 24mol H 2 O continued to react for 48h. After the reaction, the reaction solution was slowly poured into ether to obtain a white flocculent solid. Wash the product with ether and dry it, dissolve it in DMAC, pour it into a polytetrafluoroethylene mold, and wait for the solvent to evaporate and dry at 70°C to obtain polyurethane urea with high strength.
[0074] The characteristic of this embodiment is to use polycarbonate diol and alicyclic diisocyanate HMDI, chain extender diol DMBA to synthesize polyurethane urea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength at break | aaaaa | aaaaa |
| tensile strength at break | aaaaa | aaaaa |
| breaking strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com