Preparation method and application of porous carbon-based catalyst for nitrogen reduction
A catalyst and porous carbon technology, applied in the field of ammonia synthesis, can solve the problems of high cost and low catalytic activity of nitrogen reduction catalysts, and achieve the effects of enhanced nitrogen reduction performance, high catalytic activity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation method of chitin porous carbon-based nitrogen reduction catalyst:
[0063] (1) Take 3g of commercial chitin, put it in a porcelain boat, and pre-oxidize it in an air atmosphere at 250°C for 2 hours. After the reaction is completed, the temperature of the reaction system is lowered to room temperature, and the pre-oxidized chitin-based carbon material is obtained;
[0064] (2) According to ZnCl 2 、CoCl 3 and pre-oxidized chitin-based carbon material in a mass ratio of 1:3:1, in N 2 Heating to 800°C for 2 hours at a heating rate of 5°C / min under atmosphere to obtain a carbonized chitin-based carbon material;
[0065] (3) When cooling to room temperature, remove Co and Zn metal compounds in the chitin-based porous carbon with HCl with a concentration of 2M, and use deionized water to wash 5 times, and dry to obtain the chitin porous carbon-based nitrogen reduction catalyst;
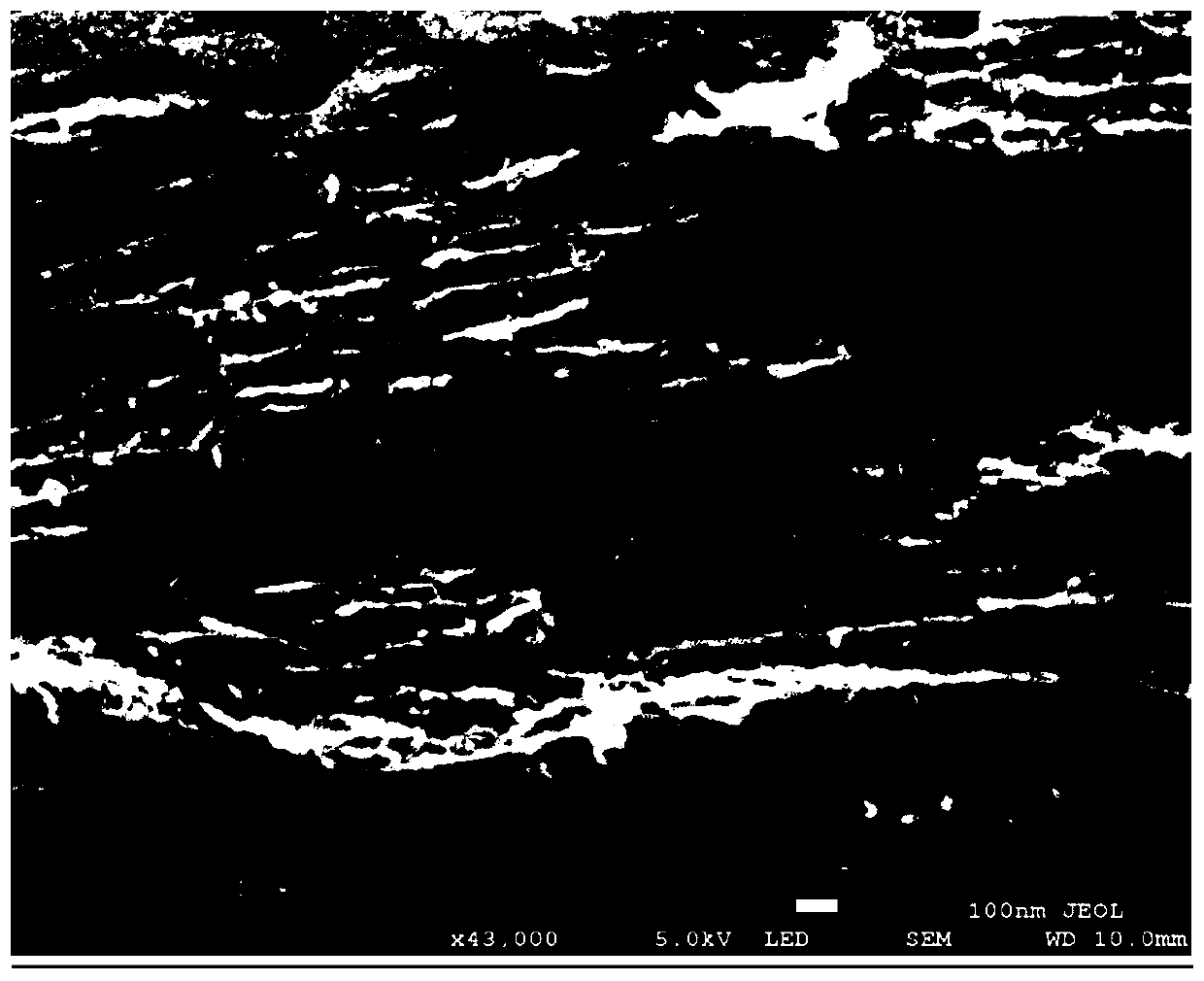
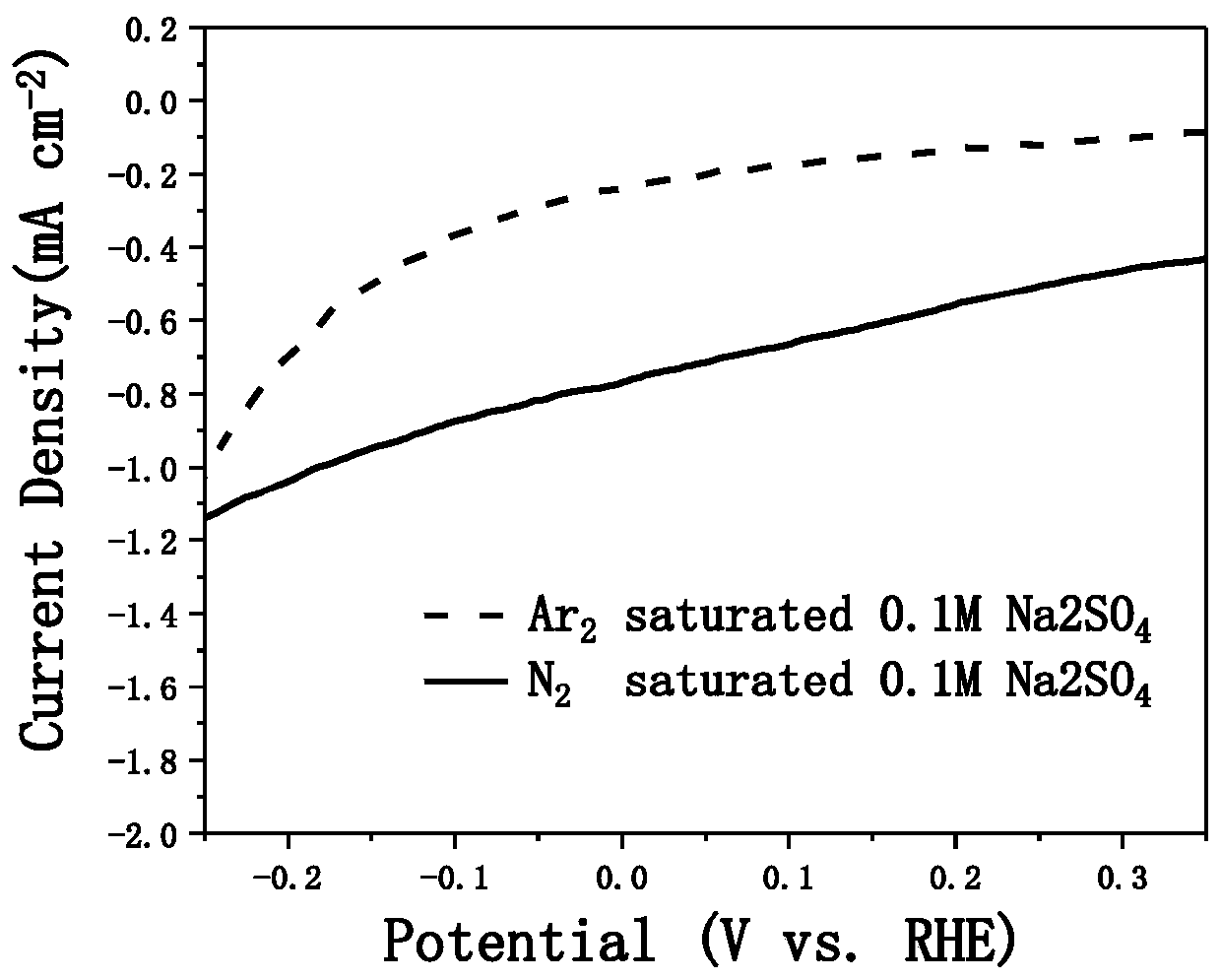
[0066] Performance test of chitin porous carbon-based nitrogen reduction cataly...
Embodiment 2
[0071] Preparation method and performance test of cyclodextrin porous carbon-based nitrogen reduction catalyst:
[0072] (1) Weigh 2g of β-cyclodextrin and 120ml of deionized water, mix and stir, put it in a 50ml polytetrafluoroethylene lining for hydrothermal pretreatment, and treat it at 220°C for 6 hours, and wait for the reaction to complete Finally, after the temperature of the reaction system drops to room temperature, the pretreated cyclodextrin-based carbon material is obtained;
[0073] (2) According to CoCl 3 Mixed with the pretreated cyclodextrin-based carbon material at a mass ratio of 1:1, at N 2 Heating to 800°C for 2 hours at a heating rate of 5°C / min under atmosphere to obtain a carbonized cyclodextrin-based carbon material;
[0074] (3) When cooling to room temperature, remove the metal compound in the cyclodextrin-based carbon material with HCl with a concentration of 2M, and wash it with deionized water for 5 times, and dry to obtain a cyclodextrin porous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com