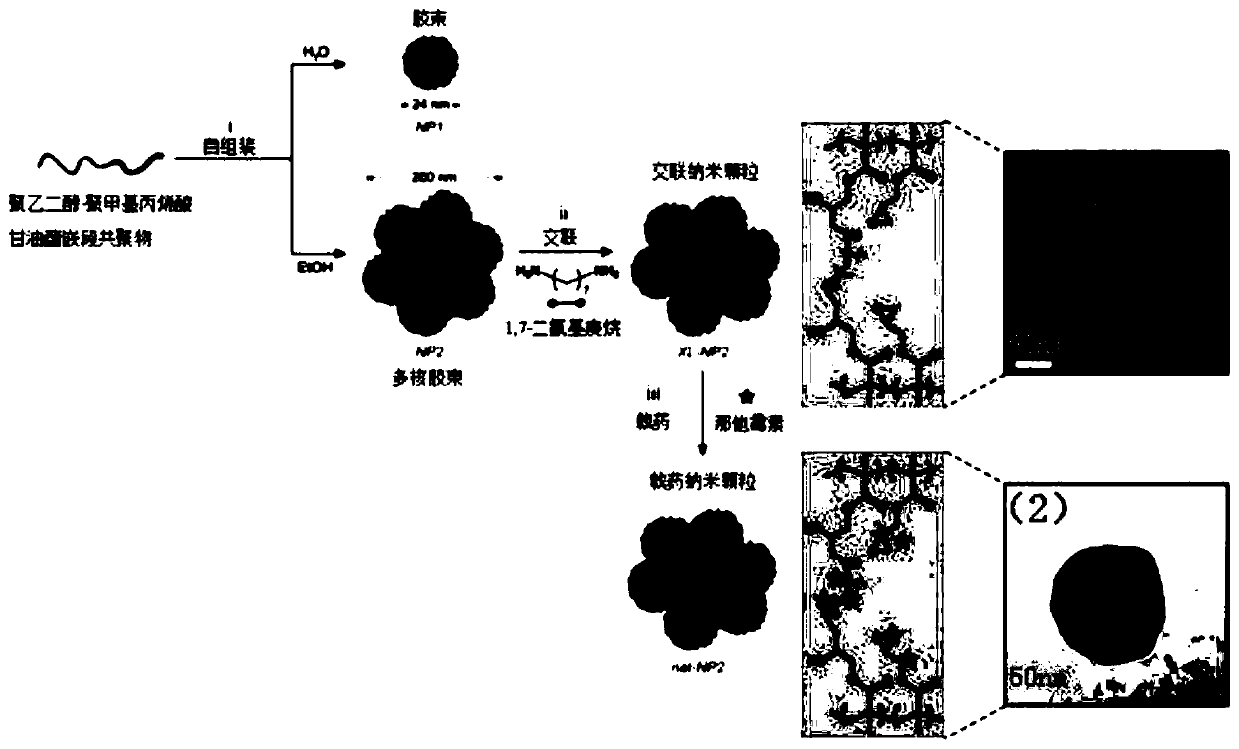
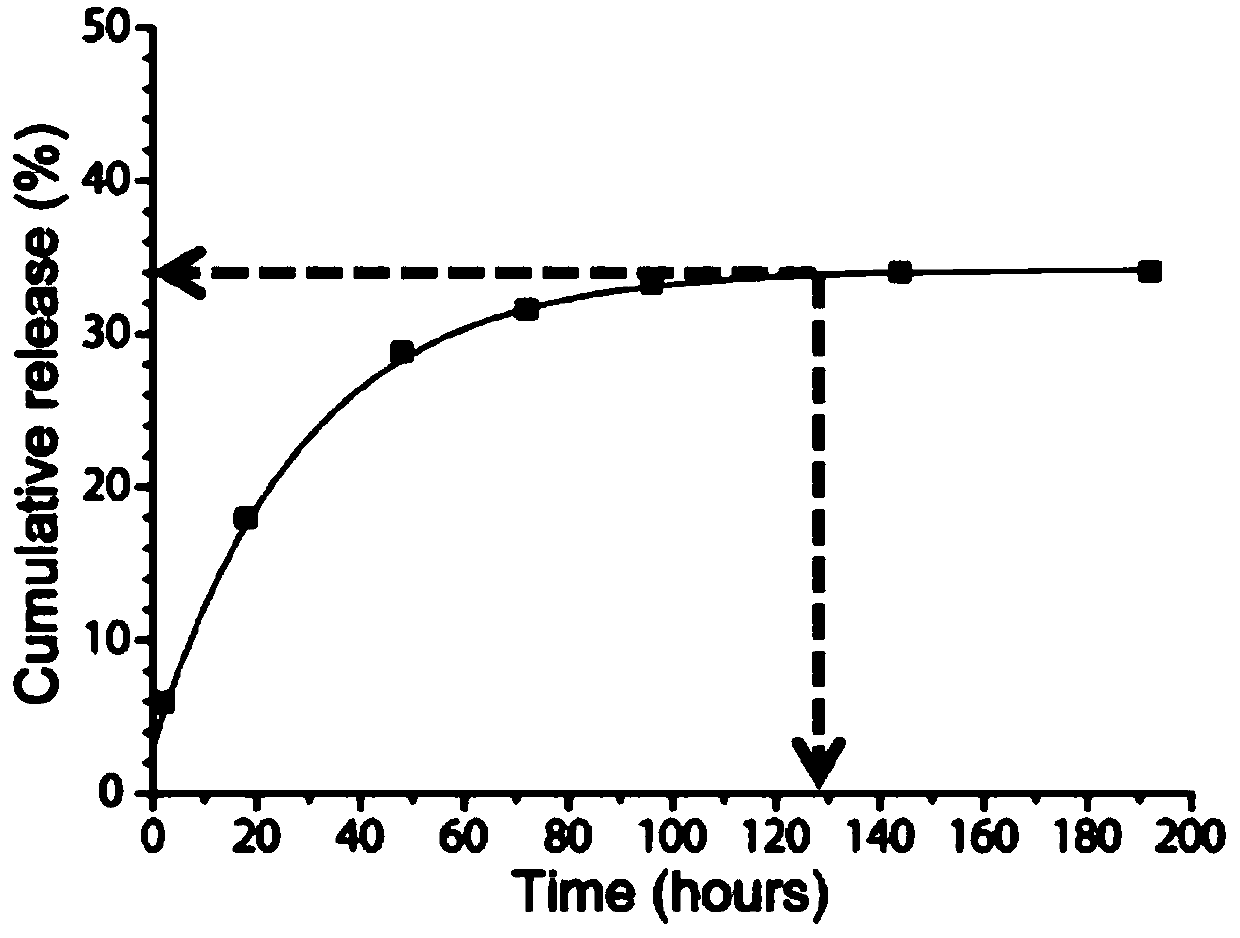
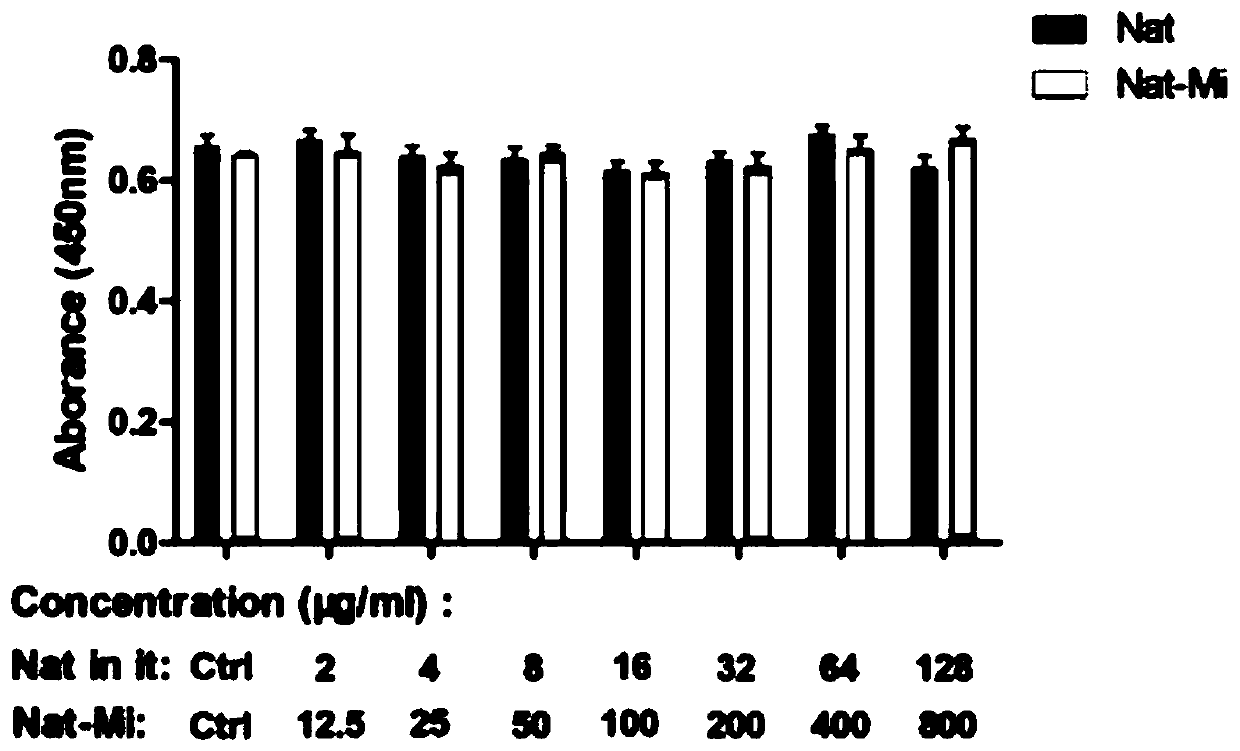
Natamycin polymer micelle eye drop and preparation method thereof
A technology of natamycin and polymer glue is applied to the field of natamycin polymer micelle eye drops and preparation thereof, which can solve the problem of high frequency of administration of natamycin eye drops, reduce the frequency of administration, The effect of solving poor compliance and prolonging the release time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]A kind of natamycin polymer micelles eye drops, comprise the natamycin polymer micelles and the phosphate buffer saline of certain mass volume ratio, wherein the natamycin accounts for in the natamycin polymer micelles The mass percentage is 10-30%, and the balance is polyethylene glycol-polyglyceryl methacrylate block copolymer.
Embodiment 2
[0040] A kind of natamycin polymer micelles eye drop, comprising the natamycin polymer micelles of 70~120mg:5mL mass volume ratio and the phosphate buffer saline solution that pH is 7.4, wherein the natamycin polymer The mass percentage of natamycin in the micelle is 10-30%, and the balance is polyethylene glycol-polyglycerol methacrylate block copolymer.
Embodiment 3
[0042] A kind of natamycin polymer micelles eye drops, comprising the natamycin polymer micelles and the phosphate buffer saline that mass volume ratio is 70mg:5mL, wherein the natamycin in the natamycin polymer micelles The mass percentage of element is 10%, and the balance is polyethylene glycol-polyglyceryl methacrylate block copolymer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com