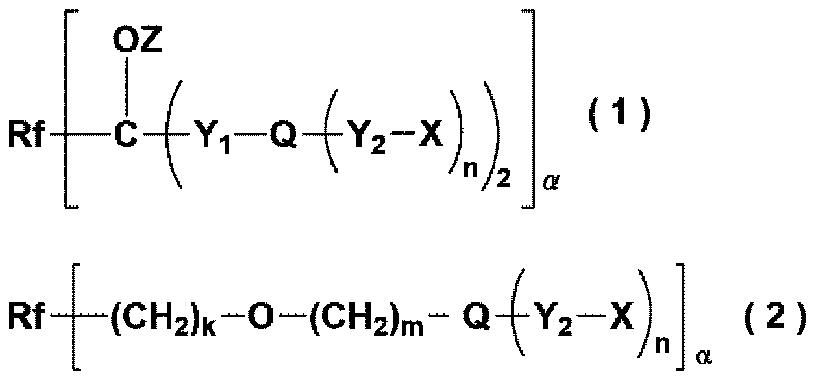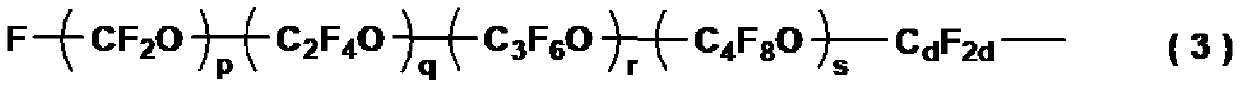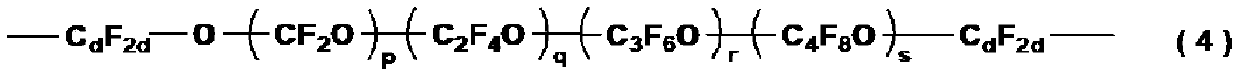Fluoropolyether group-containing polymer-modified organic silicon compound, surface treatment agent, and article
A technology of organosilicon compounds and polymers, applied in the direction of coating, etc., can solve problems such as insufficient wear resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0281] Hereinafter, synthesis examples, examples, and comparative examples are shown, and the present invention will be described in more detail, but the present invention is not limited by the following examples.
[0282] In Examples and Comparative Examples, compounds obtained in the following Synthesis Examples were used.
Synthetic example 1
[0283] [Synthesis Example 1] Synthesis of Compound 1
[0284] 150 g of tetrahydrofuran and 300 g of 1,3-bis(trifluoromethyl)benzene were mixed in a reaction vessel, and 160 ml of 0.7 M allylmagnesium bromide was added dropwise. Then, slowly drop into the following formula (A)
[0285] [Chemical 53]
[0286]
[0287] p1:q1=47:53,
[0288] Represented compound 200g (4.8×10 -2 mol), heated at 60°C for 4 hours. After heating, it was cooled to room temperature, and the solution was dropped into 300 g of a 1.2M aqueous hydrochloric acid solution to stop the reaction. After recovering the fluorine compound layer as the lower layer by a liquid separation operation, it was washed with acetone. The washed lower fluorine compound layer was collected again, and the remaining solvent was distilled off under reduced pressure to obtain a fluoropolyether group-containing polymer represented by the following formula (B).
[0289] [Chemical 54]
[0290]
[0291] p1:q1=47:53,
...
Synthetic example 2
[0318] [Synthesis Example 2] Synthesis of Compound 2
[0319] In a reaction vessel, 100 g of 1,3-bis(trifluoromethyl)benzene, 8.2 g of DBU (diazabicycloundecene) (5.4×10 -2 mol), by the following formula (B)
[0320] [Chemical 59]
[0321]
[0322] p1:q1=47:53,
[0323] Represented compound 114g (2.7×10 -2 mol) after mixing, drop 5.8g (5.4×10 - 2 mol). Next, it heated at 50 degreeC for 3 hours. After heating, it was cooled to room temperature, and aqueous hydrochloric acid solution was added dropwise. After recovering the lower fluorine compound layer by a liquid separation operation, it was washed with methanol. The washed lower fluorine compound layer was collected again, and the remaining solvent was distilled off under reduced pressure to obtain a fluoropolyether group-containing polymer represented by the following formula (E).
[0324] [Chemical 60]
[0325]
[0326] 1 H-NMR
[0327] δ0-0.2(-OSi(C H 3 ) 3 )9H
[0328] δ2.4-2.6(-C H 2 CH=CH 2 )4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com