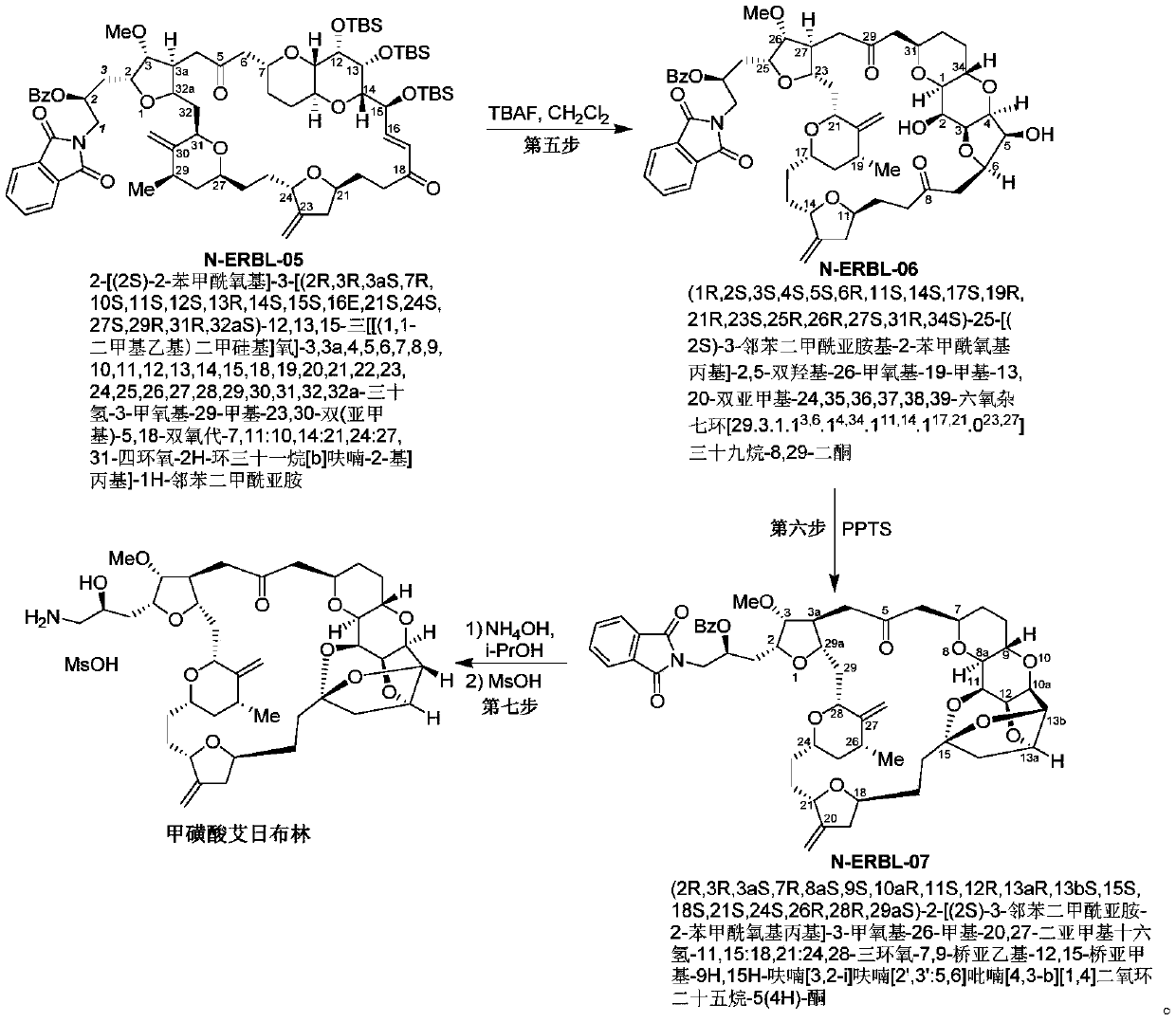
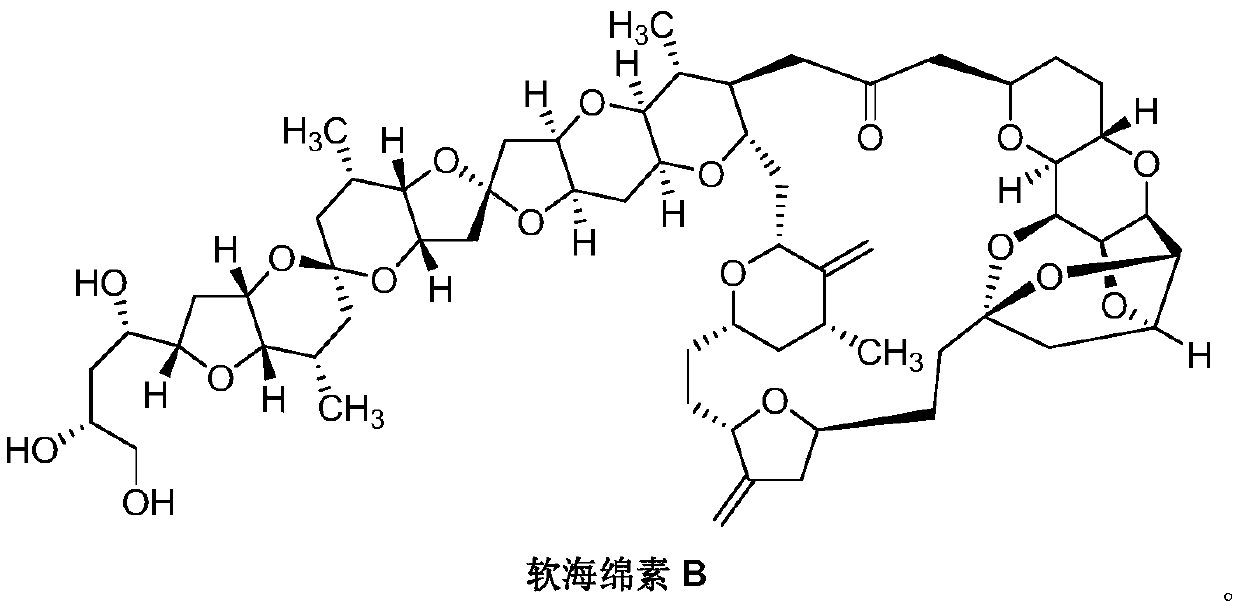
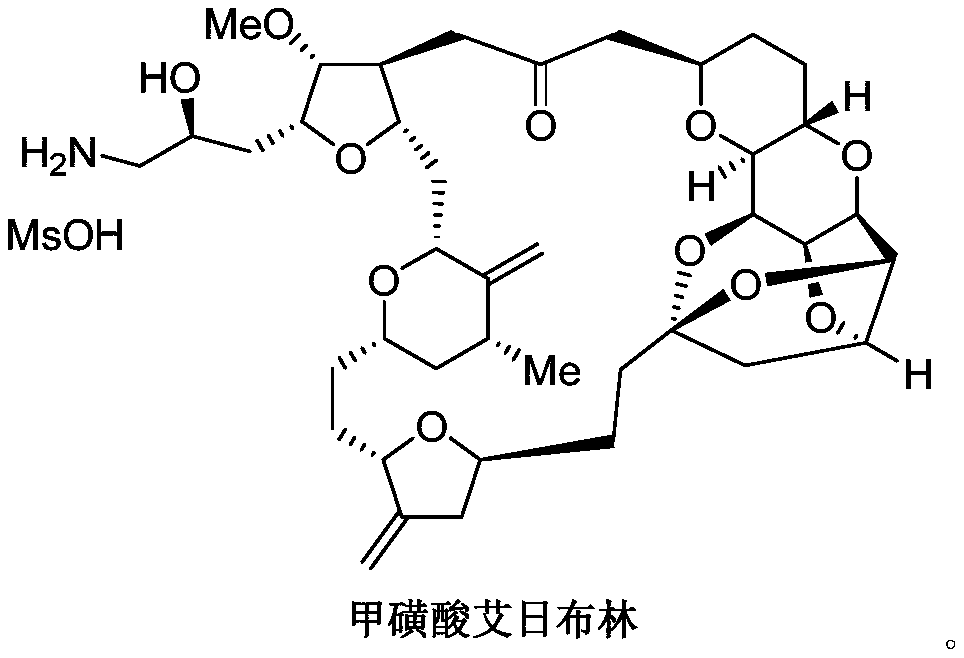
Eribulin mesylate preparation method
A technology of methanesulfonic acid and p-toluenesulfonate, which is applied in the preparation of sulfonate, bulk chemical production, organic chemistry, etc., can solve problems such as a large number of isomer impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment one: the preparation of N-ERBL-02 intermediate
[0022] Add N-ERBL-01 intermediate (25.00g, 15.37mmol) and CH 2 Cl 2 (500mL), after stirring to dissolve, add Dess-Martin oxidant (10g, 23.58mmol) in batches. After the addition, the system was stirred at room temperature until the reaction of the starting material was completely monitored by TLC. After the reaction was complete, the system was quenched by adding saturated aqueous sodium bicarbonate solution (500 mL). The system was allowed to stand, and the organic phase was separated. The organic phase was removed under low temperature and reduced pressure to remove the organic solvent, and the residue was purified by column chromatography to obtain N-ERBL-02 intermediate (20.23 g, 81.1%).
Embodiment 2
[0023] Embodiment two: the preparation of N-ERBL-03 intermediate
[0024] Sm (13.05g, 86.79mmol) and THF (500mL) were added into the four-neck flask, and diiodomethane (25.0g, 93.3mmol) was added under stirring. After the addition, the system was stirred at room temperature for 5 hours and set aside. Add N-ERBL-02 intermediate (15.05g, 9.34mmol), methanol (200mL) and THF (500mL) into another four-neck flask, stir the system and cool down to -85°C, then dropwise add the previously prepared SmI 2 solution. After the dropwise addition, the system was stirred for 4 hours until TLC confirmed that the reaction of the raw materials was complete. Add saturated K to the reaction at low temperature 2 CO 3 Aqueous solution (200 mL) was quenched. After the system was warmed up to room temperature, TBME (1 L) was added, stirred for 2 hours, allowed to stand, and the organic phase was separated. The organic solvent was removed from the organic phase under reduced pressure, and the resi...
Embodiment 3
[0025] Embodiment three: the preparation of N-ERBL-04 intermediate
[0026] Add acetonitrile (550mL), CrCl 2 (26.0 g, 211.55 mmol) and ligand (CAS: 480444-15-3, 63.2 g, 213.24 mmol). After the addition was complete, triethylamine (21.5 g, 212.5 mmol) was slowly added dropwise while the system was stirred. After the addition is complete, keep stirring at 30-35°C for 2 hours, then add NiCl 2 (2.75 g, 21.1 mmol), stirred. Then a solution of N-ERBL-03 intermediate (22.5 g, 15.33 mmol) in THF (500 mL) was added dropwise to the reaction system at room temperature. After the dropwise addition, the system was stirred at room temperature for 5 hours until the reaction of the raw materials controlled by TLC was completed. The reaction system was quenched by adding water (300mL), the system was filtered, the filtrate was extracted three times with n-heptane, the organic phase was combined, the organic phase was decompressed to remove the organic solvent, and the residue was purified ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com