Vaccine against necrotic enteritis in poultry
A poultry, vaccine technology, applied in] this application relates to the preparation of poultry necrotic enteritis, in another aspect, Clostridium perfringens pilus polypeptide, in one aspect provides an isolated gas-producing Clostridium perfringens fimbriae, polypeptides for vaccines, vaccines for necrotizing enteritis, say, immunogenicity field as described herein, able to resolve the strains that work, necrotizing enteritis non-specific, unknown necrotizing enteritis Pathogenesis and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0068] Other features of the invention will become apparent from the following non-limiting examples, which illustrate by way of example the principles of the invention.
example 1
[0069] Example 1: Production of Purified Recombinant Pili-Associated Polypeptides from Clostridium perfringens
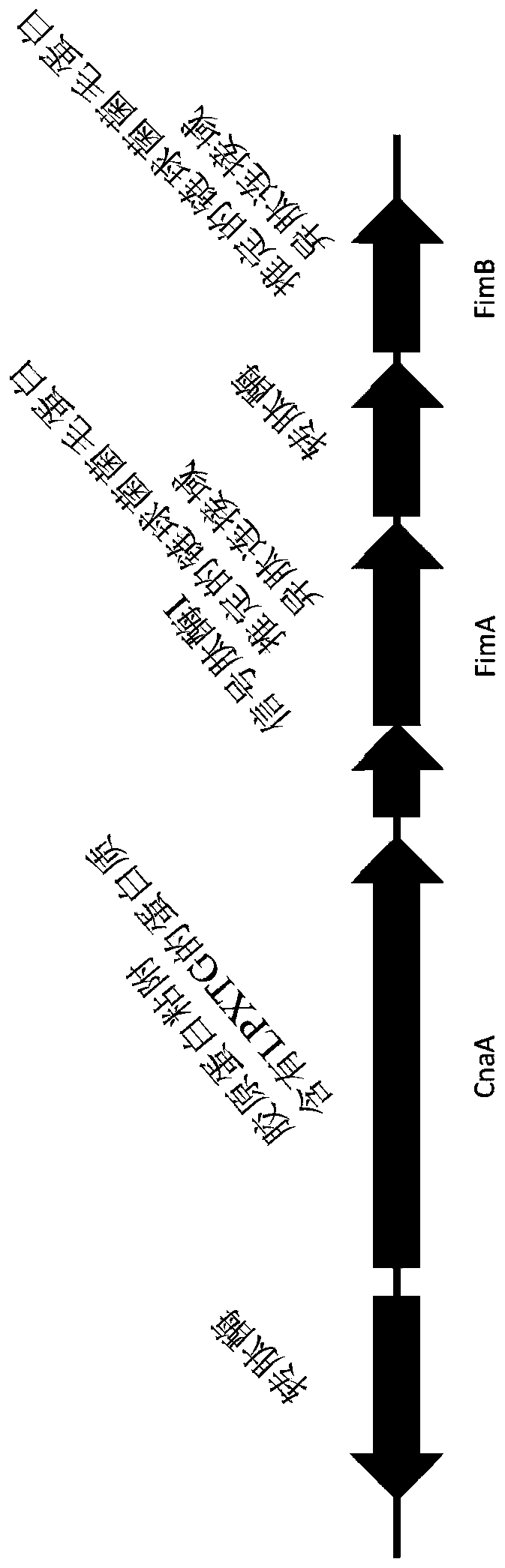
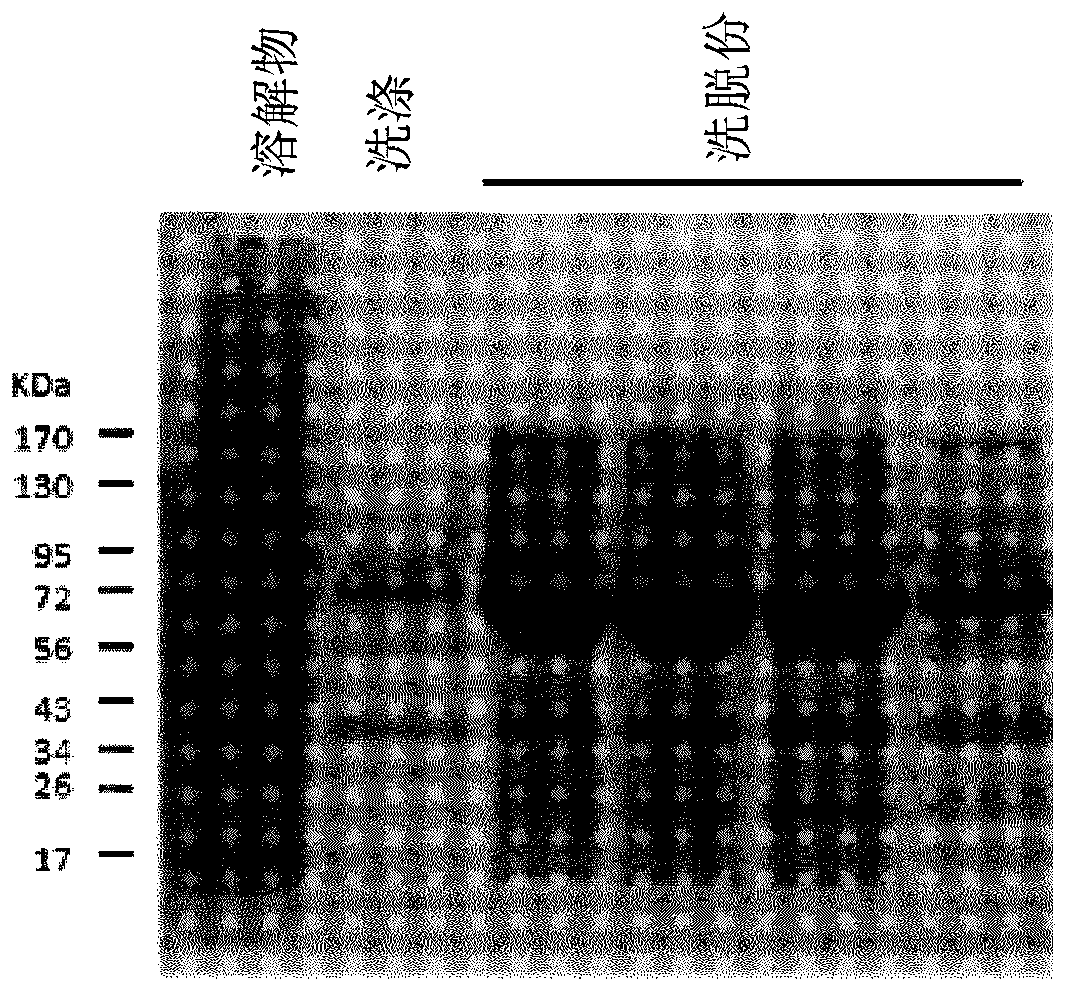
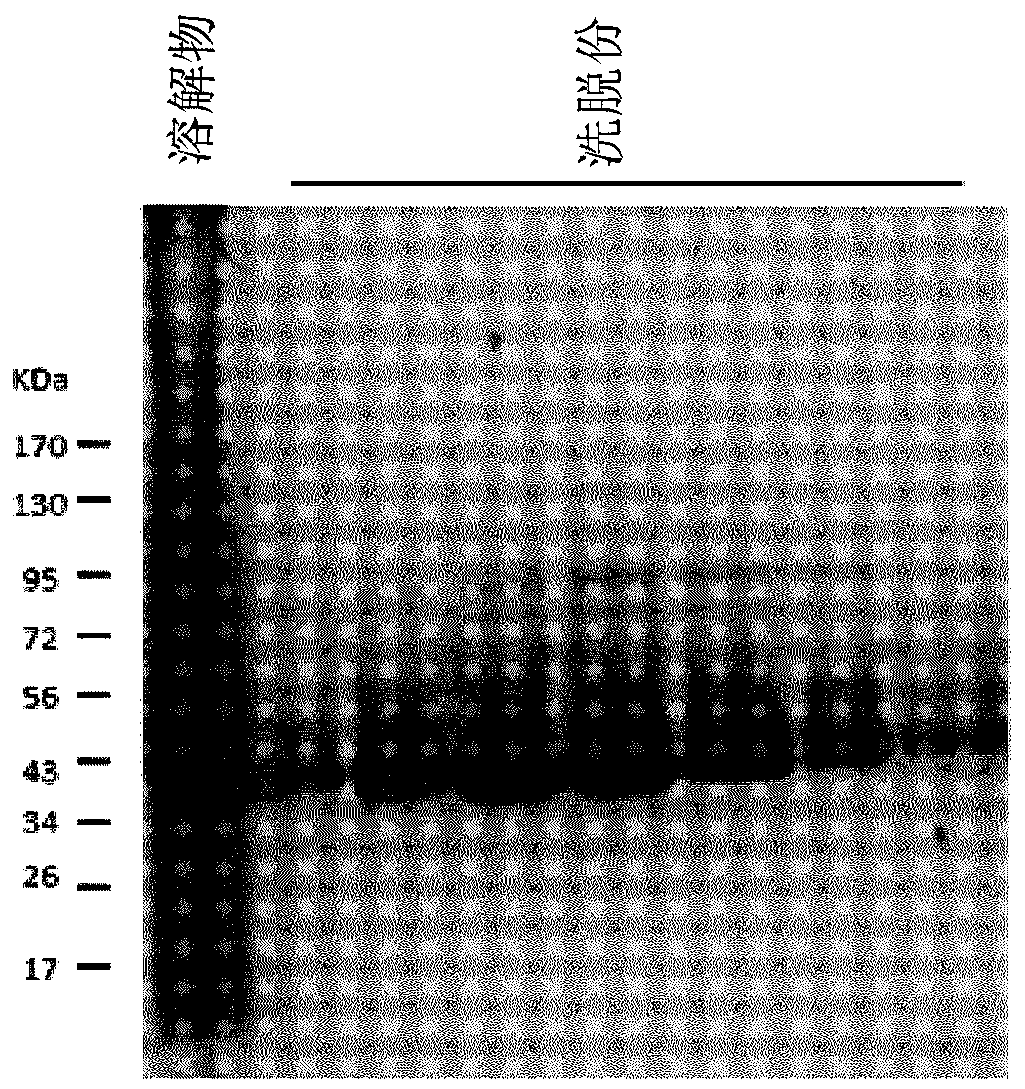
[0070] The coding regions of the three pilus subunits (cnaA, fimA and fimB) were codon optimized and truncated to exclude the predicted N-terminal signal peptide and C-terminal cell wall sorting signal LPXTG transmembrane domains. The C-terminal domain is a hydrophobic region predicted to be removed during pili assembly. Truncated codon-optimized coding regions were synthesized (Integrated DNA Technologies, Coralville, USA) using In-Fusion TM Cloning, cloned into pET28a expression vector (MilliporeSigma, Ontario, Canada) according to the manufacturer's instructions (Takara Bio USA, Mountain View, California, USA) Sequence verification was performed in Etobicoke, Ontario, Canada, and then transformed into E. coli BL21 cells. Transformed colonies were grown in 1 L of LB broth supplemented with 50 μg / ml kanamycin and 1 mM IPTG at 37° C. for 18 hours with shaking. Th...
example 2
[0082] Example 2: Preparation of Clostridium perfringens strain CP1 pili subunit knockout mutants
[0083] It is generally as described above (Yu Q. (Yu, Q.), Lepp D (Lepp, D.), Mehdizadeh Gohari I. (Mehdizadeh Gohari, I.), Wu T. (Wu, T.), Zhou H.(Zhou,H.), Yin X.(Yin,X.), Yu H.(Yu,H.), Prescott J.F.(Prescott,J.F.), Nie S.P.(Nie, S.P.), Xie M.Y. (Xie, M.Y.), Gong J. (Gong, J.), 2017, The pathogenesis of Clostridium perfringens-induced necrotic enteritis in poultry requires the Agr-like quorum sensing system (TheAgr-like quorum sensing system is required for necrotic enteritis pathogenesis in poultry caused by Clostridium perfringens), Infection and Immunity (Infection and Immunity) 85(6):e00975-16), induced by ClosTron mutation (Heap J.T. (Heap, J.T.) et al., Molecular Biol (Methods Mol.Biol.)(2010),646:165-182), the three pili subunit genes (cnaA, fimA and fimB) in the virulent Clostridium perfringens strain CP1 were inserted into ) were each inactivated to generate CP1 kno...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com