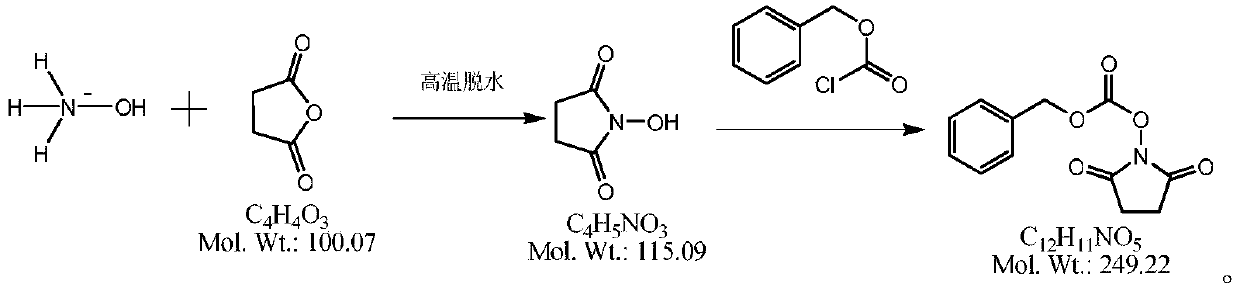
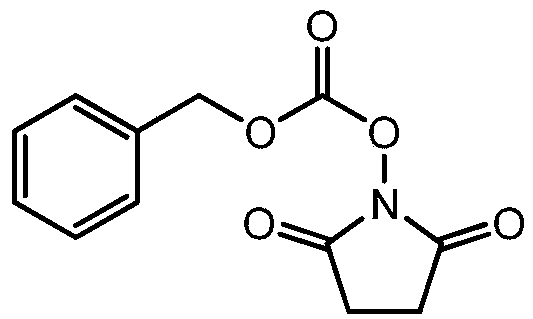
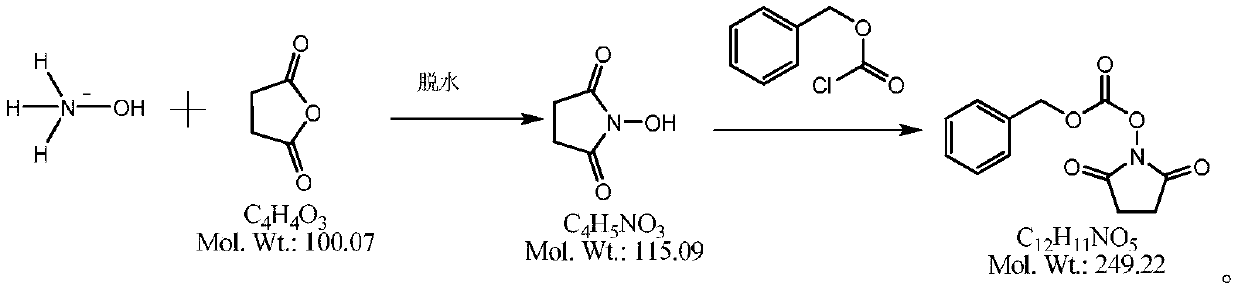
Method for synthesizing N-(carbobenzoxy) succinimide by one-pot two-phase method
A technology of succinimide and benzyloxycarbonyl is applied in the field of one-pot two-phase synthesis of N-succinimide, which can solve the problems of high process cost, cumbersome synthesis steps, and high purity requirements of raw materials, so as to reduce the operation steps , The effect of reducing the process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 1000ml four-necked flask, add 100g of water, under stirring, add 125g of hydroxylamine sulfate (molecular weight 164.1, 0.76mol), dropwise add 200g of liquid caustic soda (molecular weight 40.0, 2mol) with a mass fraction of 40%, under stirring, drop into Diacid anhydride 152.3g (molecular weight 100.1, 1.52mol), all dissolved under stirring, add 5g of 85% phosphoric acid (molecular weight 98, 0.043mol), vacuum dehydration, control the temperature in the bottle is not higher than 160 ℃, until anhydrous comes out, cool down to Below 100°C, add 250g of toluene, heat up to reflux with stirring, reflux to separate water until no water comes out, and drop to 25-30°C. 500g of liquid caustic soda aqueous solution (mass fraction is 40% liquid caustic soda 100g, water 400g) is added dropwise in the reaction bottle, at this moment, the solid in the bottle disappears, and it is a two-phase distribution. With rapid stirring, 150g of benzyl chloroformate (molecular weight 170.6 ...
Embodiment 2
[0027] In a 1000ml four-neck flask, add 100g of water, under stirring, add 105g of hydroxylamine hydrochloride (molecular weight 164.1, 0.64mol), dropwise add 250g of liquid caustic soda (molecular weight 40.0, 2.5mol) with a mass fraction of 40%, and under stirring, drop in 152.3 g of succinic anhydride (molecular weight 100.1, 1.52 mol), stir to dissolve completely, add 5 g of sulfuric acid (molecular weight 98, 0.05 mol) with a mass fraction of 98%, vacuum dehydration, control the temperature in the bottle not higher than 160 ° C, until anhydrous Remove, lower the temperature to below 100°C, add 250g of toluene, raise the temperature to reflux with stirring, reflux to separate water until no water comes out, and drop to 25-30°C. Add 500 g of sodium carbonate aqueous solution (sodium carbonate 150 g, water 350 g) dropwise in the reaction bottle, at this moment, the solid in the bottle disappears, and it is a two-phase distribution. Quickly stir and dropwise add 130 g of benzy...
Embodiment 3
[0029] In a 1000ml four-necked flask, add 100g of water, under stirring, add 125g of hydroxylamine sulfate (molecular weight 164.1, 0.76mol), dropwise add 200g of liquid caustic soda (molecular weight 40.0, 2mol) with a mass fraction of 40%, and under stirring, drop into Diacid anhydride 145g (molecular weight 100.1, 1.45mol), stir to dissolve completely, add 85% phosphoric acid 5g (molecular weight 98, 0.043mol), vacuum dehydration, control the temperature in the bottle not higher than 160 ℃, until anhydrous, drop to 100 Below ℃, add 200g of toluene, raise the temperature to reflux under stirring, reflux to separate water until no water is separated, and drop to 25-30 ℃. Add 500 g of liquid caustic soda aqueous solution (mass fraction is 40% liquid caustic soda 100 g, water 300 g) dropwise in the reaction bottle, at this moment the solid in the bottle disappears, and it is a two-phase distribution, quickly stir and dropwise add 150 g of benzyl chloroformate with a molecular we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com