Application of three-dimensional transitional metal nanoarray used as catalyst for hydrogen production from hydroboron
A technology of transition metals and nano-arrays, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of reducing catalyst usage efficiency, difficult to control hydrolysis reaction, difficult to separate and recycle, etc., to achieve excellent Catalytic activity and stability, promotion of efficient transport, and efficient hydrolysis for hydrogen production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Step 1: Add 20 mL of distilled water to the PTFE liner, then add 0.291 g of cobalt nitrate, 0.093 g of ammonium fluoride and 0.30 g of urea and stir until the solids are completely dissolved to form a transparent solution.
[0032] Step 2: Put the titanium mesh into the inner lining of the reactor in step 1, seal the polytetrafluoroethylene inner lining into a stainless steel mold, put it in a constant temperature drying oven under closed conditions, and heat and react at 120° C. for 6 hours.
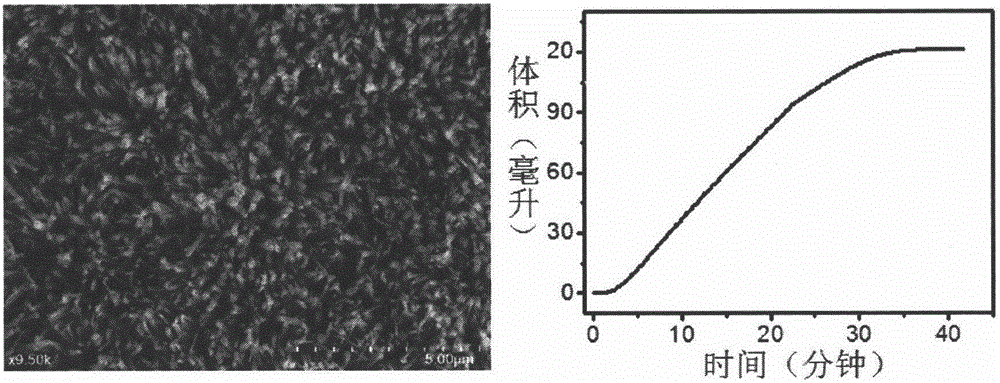
[0033] Step 3: After the reaction is completed, the titanium mesh is taken out, washed with distilled water and absolute ethanol in turn, and the washed titanium mesh is annealed at 400°C for 3 hours in an air atmosphere to obtain a three-dimensional Co 3 o 4 nanowire array ( figure 1 ).
[0034] Step 4: Select the concentration of the sodium borohydride alkaline solution to be 1 wt%, wherein the concentration of sodium hydroxide is 1 wt%, and the total volume of the solution i...
Embodiment 2
[0036] Step 1: Add 36 mL of distilled water to the PTFE liner, add 1.45395 g of nickel nitrate and 1.4019 g of hexamethylenetetramine, and stir until the solids are completely dissolved to form a transparent solution.
[0037]Step 2: Put the carbon fiber cloth into the inner lining of the reactor in step 1, seal the polytetrafluoroethylene inner lining into a stainless steel mold, place it in a constant temperature drying oven under closed conditions, and heat it at 100°C for 10 hours.
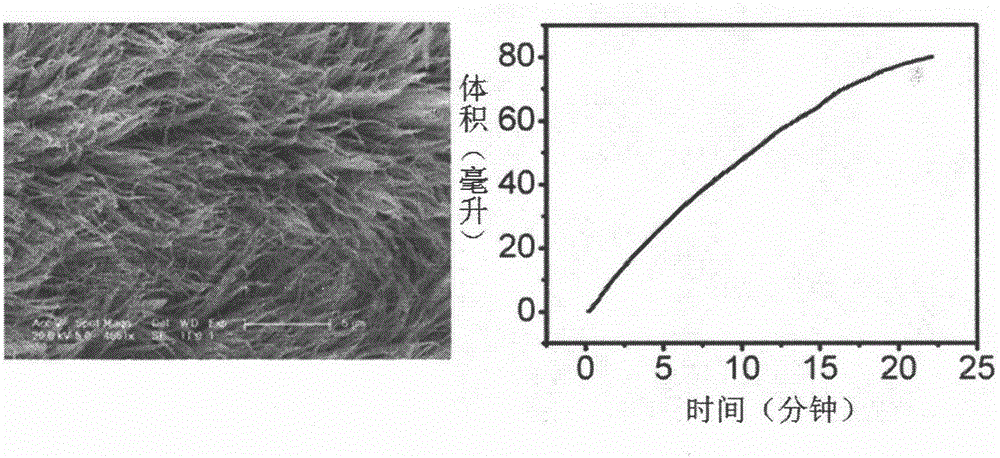
[0038] Step 3: After the reaction is completed, take out the carbon fiber cloth, wash it, arrange the washed carbon fiber in a vacuum drying oven and dry it in vacuum at 40°C for 24 hours to obtain a nickel hydroxide nanosheet array structure, and then place it in an air atmosphere , and annealed at 400 °C for 2 h to obtain a three-dimensional NiO nanosheet array.
[0039] Step 4: Select the concentration of the sodium borohydride alkaline solution to be 5wt%, wherein the concentration of sodi...
Embodiment 3
[0041] Step 1: Add 35mL of distilled water to the polytetrafluoroethylene lining, add 0.87g of cobalt nitrate, 0.11g of ammonium fluoride and 0.18g of urea to each milliliter of water, stir and dissolve to form a transparent solution.
[0042] Step 2: Put the silicon wafer into the lining of the reaction kettle in Step 1, seal the polytetrafluoroethylene lining into a stainless steel mold, place it in a constant temperature drying oven under closed conditions, and heat and react at 120°C for 12 hours.
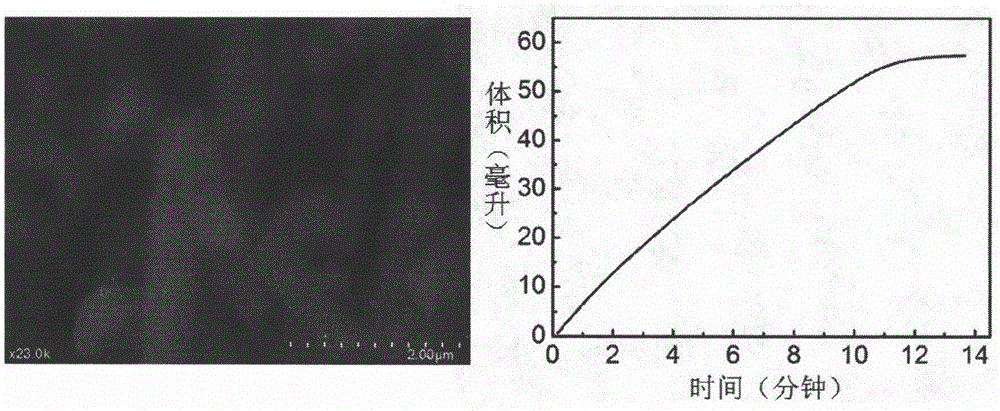
[0043] Step 3: After the reaction is completed, the silicon wafer is taken out, cleaned, and the washed silicon wafer is placed in a vacuum drying oven and vacuum-dried at 40° C. for 24 hours to obtain a Co(OH)F nanowire array structure, and then in Annealed at 420 °C for 2 h in an ammonia atmosphere to obtain a three-dimensional Co 4 N nanowire arrays.
[0044] Step 4: Select the concentration of the sodium borohydride alkaline solution to be 10wt%, wherein the concentration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com