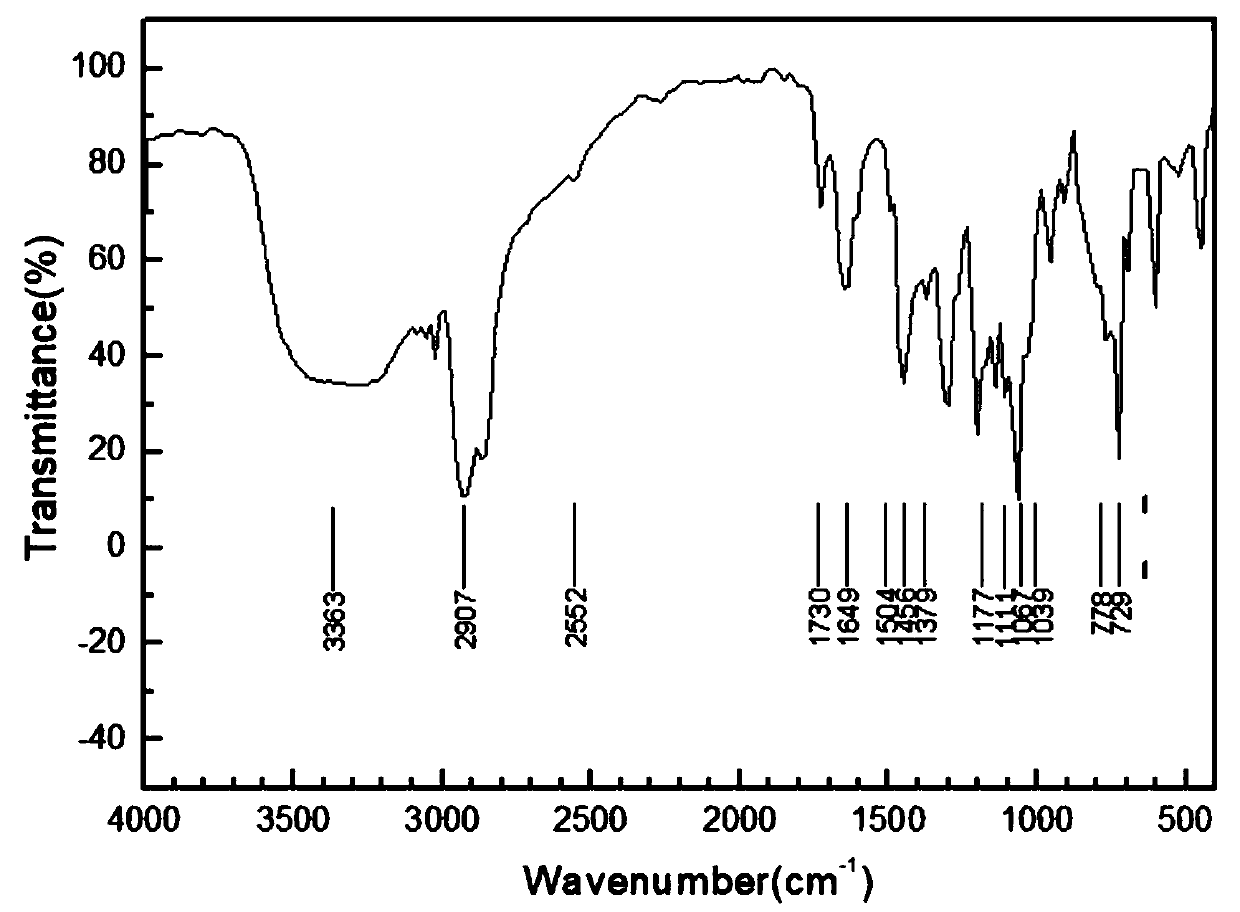
Resource treatment process for inorganic salt hazardous wastes
A treatment process and inorganic salt technology, applied in the field of inorganic salt hazardous waste resource treatment process, can solve the problems of adsorbent loss, difficult treatment, high adsorption cost, etc., increase specific surface area, improve adsorption effect, and solve environmental protection problems Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A resource treatment process for inorganic salt hazardous waste, and its technical scheme includes the following steps:
[0031] Step 1. Feeding treatment. Firstly, the slag salt is transported to the pretreatment plant, mixed in the mixing tank, and then crushed by the crusher and transported to the feed hopper, and then sent to the negative pressure by the closed screw conveyor The dryer is dried at 150℃ to control the moisture content of the slag salt at 0.1%;
[0032] Step two, high temperature oxidation, the dried slag salt enters the oxidation furnace, and is calcined at a high temperature of 450°C for 60 seconds, and then is further oxidized and calcined in a rotary oxidation furnace;
[0033] Step three, waste salt refining treatment, the high-temperature oxidized coarse salt is sent to the dissolving kettle through a screw conveyor, water is added to the dissolving kettle, the coarse salt is prepared into 310g / L saturated brine with a temperature of 50℃, and the solut...
Embodiment 2
[0042] A resource treatment process for inorganic salt hazardous waste, and its technical scheme includes the following steps:
[0043] Step 1. Feeding treatment. Firstly, the slag salt is transported to the pretreatment plant, mixed in the mixing tank, and then crushed by the crusher and transported to the feed hopper, and then sent to the negative pressure by the closed screw conveyor The dryer is dried at 180°C, and the moisture content of the slag salt is controlled at 0.5%.
[0044] Step two, high temperature oxidation, the dried slag salt enters the oxidation furnace, and is calcined at a high temperature of 480°C for 90 s, and then is further oxidized and calcined in a rotary oxidation furnace;
[0045] Step three, waste salt refining treatment, the high-temperature oxidized coarse salt is sent to the dissolving kettle through a screw conveyor, water is added to the dissolving kettle, and the coarse salt is made into 450g / L saturated brine with a temperature of 505°C and disso...
Embodiment 3
[0054] A resource treatment process for inorganic salt hazardous waste, and its technical scheme includes the following steps:
[0055] Step 1. Feeding treatment. Firstly, the slag salt is transported to the pretreatment plant, mixed in the mixing tank, and then crushed by the crusher and transported to the feed hopper, and then sent to the negative pressure by the closed screw conveyor The dryer is dried at 250°C to control the moisture content of the slag salt at 1%;
[0056] Step 2: High-temperature oxidation, the dried slag salt enters the oxidation furnace, and is calcined at a high temperature of 500°C for 120s, and then undergoes further oxidation and calcining treatment in a rotary oxidation furnace;
[0057] Step three, waste salt refining treatment, the high-temperature oxidized coarse salt is sent to the dissolving kettle through a screw conveyor, water is added to the dissolving kettle, the coarse salt is prepared into saturated brine at 650g / L and the temperature is 60℃,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com